While scientific progress hinges on data integrity, plagiarism, data fabrication and image manipulation and other biomedical research scandals are ongoing concerns. An article in Science recently made waves by revealing startling conclusions from the research of the German neuropsychologist Bernhard Sabel, who developed a fake-paper detector. Publishing his findings in a preprint, Sabel concluded that there…
AI in drug development: Janssen exploring potential of AI in everything from target discovery to clinical trials
As enterprise AI adoption surges, the life science industry stands at a pivotal juncture. “We are truly at a tipping point,” remarked Najat Khan, chief data science officer at Janssen, during the 2023 Stanford Drug Discovery Symposium. “It’s amazing how quickly we’ve been able to make progress across the whole value chain in terms of…
Accelerating Alzheimer’s research: ADDF’s chief scientific officer reflects on the Lauder Foundation’s $200M gift
The Estée Lauder family has donated $200 million to the Alzheimer’s Drug Discovery Foundation (ADDF), a nonprofit they founded in 1998 to support Alzheimer’s research. The gift is the largest ADDF has received. “We’ve deployed about $270 million so far for about 700 programs in 19 countries of drug discovery and development over the past…
AbbVie and Janssen voluntarily withdraw Imbruvica from accelerated approvals for MCL and MZL
AbbVie (NYSE:ABBV) and Janssen Pharmaceutical (NYSE:JNJ) have revealed their intent to voluntarily withdraw the accelerated approvals for Imbruvica (ibrutinib) for patients with mantle cell lymphoma (MCL) and marginal zone lymphoma (MZL) in the U.S. Ibrutinib is a selective Bruton’s tyrosine kinase (BTK). The main reasons for the move relate to FDA’s request for additional studies…
Three strikes in pharma: Exploring recent drug withdrawals and clinical trial challenges
Pharmaceutical companies face a long list of regulatory challenges ranging from patent expiry to bioequivalence and international harmonization. It’s not uncommon for drug makers to withdraw or discontinue drugs after failing to meet clinical requirements or endpoints, resulting in drug withdrawals. On average, life science companies pull roughly 4,500 drugs and devices from the market,…
Incorporating real-world evidence into the life cycle of drug development
Utilizing real-world data (RWD) to generate real-world evidence (RWE) is not new and pharmacoepidemiologists have long-standing experience designing and implementing studies utilizing RWE to satisfy regulatory post-marketing safety commitments and to evaluate the effectiveness of drugs. However, RWD and RWE can be used more largely to support the full life cycle of drug development, during…
Breaking down barriers: Prioritizing diversity in clinical trials
Diversity in clinical trials is crucial for ensuring that new medical treatments are safe and effective for all populations. Last year, the FDA released draft guidance that aims to improve the enrollment of historically underrepresented populations in clinical trials. Demand is growing for more transparency and diversity in clinical trials, according to Erin Leckrone, senior…
Best practices for data capture in long-term follow-up studies
Clinical trials and long-term follow-up studies (LTFUs) hinge on a whirlwind of data collection, where the volume and quality of patient data form the cornerstone of success. Erratic and patchy data can throw a monkey wrench into evaluating the potency and safety of cutting-edge drugs and devices. Such snags can drag approvals through the mud…
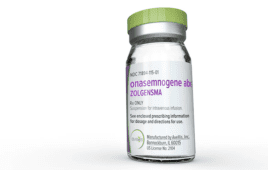
One-time gene therapy Zolgensma from Novartis shows lasting benefits for SMA patients
A gene therapy for spinal muscular atrophy (SMA) patients is making waves. Novartis (NYSE:NVS) revealed in a press release new long-term data highlighting the durability of Zolgensma (onasemnogene abeparvovec) up to 7.5 years after a single treatment. The data comes from two long-term follow-up studies, LT-001 and LT-002, which examined a range of patient populations…
How EEG and machine learning are transforming epilepsy clinical trials
Epilepsy is a brain disorder that triggers recurring seizures. It is the fourth the most common neurological disorders in the world, according to the Epilepsy Foundation. The Centers for Disease Control and Prevention estimates that 65 million people worldwide have active epilepsy. In 2015, 1.2% of the total U.S. population — 3 million adults and…
Harnessing the untapped potential of legacy data in pharma R&D
Clinical trials for a new therapy cost a median of $41,117 per patient. Costs like this are no surprise to pharma leaders. But during an age of increasing budgetary pressures, drug developers are under pressure to do more with less money and staff. While there are no “simple” answers to this challenge, there is one strategy that offers…
Janssen sees Blood Cancer Awareness Month as an opportunity to close the clinical trial diversity gap
Janssen (NYSE:JNJ) is working to highlight the importance of cancers such as leukemias, lymphomas and multiple myeloma for Blood Cancer Awareness Month in September. Some 1.5 million people in the U.S. are currently living with or in remission from blood cancers, according to the Leukemia & Lymphoma Society. Roughly 35,000 people are diagnosed with multiple myeloma…
FDA releases draft guidance to protect children in clinical trials
FDA has published draft guidance to clarify its perspective on including children in clinical trials. The agency notes that it wrote the draft guidance to help industry, sponsors and institutional review boards (IRBs) protect children in clinical studies testing drugs, biological products and medical devices. The draft guidance is titled “Ethical Considerations for Clinical Investigations of Medical…
Defining complexity: A framework to identify complex clinical trials and set them up for success
It’s easy to feel like clinical trials are complex. They are experiments involving human beings — so things change. But, despite feeling like complexity is everywhere, there is little clarity on what constitutes a complex trial and what sponsors need to do to prepare these trials for success. Both the FDA and EMA, for example,…
What biopharmas should know about RBM and RBQM
As a growing number of emerging biopharma companies seek to develop novel treatments for chronic diseases, the clinical trials market could be worth $78.3 billion by 2030. Clinical trials, however, continue to be difficult and expensive to manage. Yet successful clinical trials, however, remain critical for investors. Small biopharmas are thus looking for guidance in navigating…
Startup vies to get blockchain adoption in pharma off the ground
Blockchain may be arguably one of the most hyped technologies in recent memory, but the distributed ledger technology better known for its role in cryptocurrency may have significant potential in the pharma supply chain, clinical trials and beyond. A blockchain-based initiative known as PharmaLedger has won support from prominent companies like Pfizer, Novartis, Merck &…
A real-world data approach for bridging diversity disparities in clinical trials
The lack of appropriate representation in clinical trials, particularly in terms of ethnicity and race, has been a long-standing issue that directly impacts health equity and treatment efficacy. In a 2020 analysis of the global participation in clinical trials, the Food and Drug Administration (FDA) highlighted the vast difference between enrolled participants and the global population.…
Data-driven diversity: Making clinical trials equitable for all
Lack of diversity in clinical trials has long been an issue, driven by challenges with recruitment and participation. In recent years, pharmaceutical companies have prioritized recruiting more diverse patient groups for their trials. And in some areas, it is working. In the past ten years, the representation of Black and African American patients in U.S.-based…
Verana Health aims to go deep with its real-world data network
The task of extracting health insights from electronic health records (EHRs) has taken longer to materialize than some pundits projected a decade or so ago. But the situation is quickly changing with continued advances in data science, said Sujay Jadhav, CEO of Verana Health (San Francisco), which has created a real-world data network in partnership…
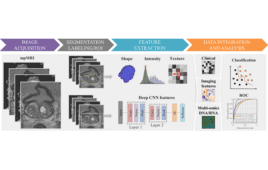
Radiomics: The present and future of advanced imaging analytics
Radiomics, or the science of advanced imaging analytics, is an emerging field that promises more personalized care, improved clinical decision support and greater efficiency in clinical trials. Radiomics are being used to discover new biomarkers, improve the accuracy of diagnosis, predict the risk of disease and likelihood of treatment response, and identify clinical endpoints. Today,…
9 predictions for pharma in 2022
The pharma industry has been slower to embrace technologies such as AI and digital technology than many less-regulated sectors. The COVID-19 pandemic has changed the equation, forcing pharma companies to become more agile and open-minded in approaching drug discovery and development, including managing evolving clinical trials. In this article, several experts offered their predictions of…
RedHill Biopharma makes progress in RHB-107 COVID-19 study
RedHill Biopharma Ltd. (NSDQ:RDHL) has randomized the last patient in Part A of an ongoing Phase 2/3 study focused on RHB-107 (upamostat), an investigational antiviral. The Tel Aviv–based company anticipates that RHB-107, a serine protease inhibitor, would be broadly effective against emerging SARS-CoV-2 variants. RedHill Biopharma expects top-line data for Part A of the trial,…
U.S. to buy 1.4 million additional courses of molnupiravir
Merck (NYSE:MRK) and Ridgeback Biotherapeutics appear to be gaining growing support for their oral COVID-19 therapy molnupiravir. One week after Great Britain granted conditional marketing authorization for the drug, the companies announced that the U.S. government intends to purchase 1.4 million additional medicine courses for approximately $1 billion. The U.S. has committed to buy approximately 3.1…
Tightening regulations in Japan drive PMDA compliance with integrated pharmacovigilance ecosystems
Pharmaceutical regulations in Japan are continuously expanding and updating to improve the safety of pharmaceutical drugs. Throughout history, the detection and understanding of harmful side effects of pharmaceutical drugs have led to new enhancements of regulations to allow regulatory agencies to find adverse events more effectively. In turn, this has led to stricter regulations. Likewise,…
Merck projects at least $5B in 2022 sales for molnupiravir
Merck’s (NYSE:MRK) investigational oral COVID-19 drug could net between $5 billion and $7 billion in revenue through the end of 2022 if FDA authorizes its use in December, the company projected. Sales of the drug could be higher, assuming it finds widespread use in wealthy countries. Merck expects sales between $500 million and $1 billion…