Johnson & Johnson’s nipocalimab, which works by targeting the neonatal Fc receptor (FcRn), has the potential to treat an array of autoimmune conditions. But the antibody recently hit a snag in the first-ever clinical study of an FcRn inhibitor in rheumatoid arthritis (RA), missing its primary endpoint. The development has sparked debate within the rheumatology…
Cybin sees near future for psychedelic therapy after promising interim phase 2 data
With promising interim phase 2 data in hand, Cybin believes psychedelic therapy will become a reality in the “not too distant future,” according to CEO Doug Drysdale. As recently as the 1990s, it would be difficult to imagine that a psychedelic drug would potentially be a clinical option for a mood disorder like depression. But…
The future of MDD treatment: A comparative table highlighting the emergence of fast-acting therapies
The treatment landscape for major depressive disorder (MDD) continues to diversify, and in the coming years, psychedelic options may be available, including COMPASS Pathways COMP360 and the deuterated psilocybin analog CYB003 from Cybin. CYB003 demonstrated a significant -14.08 point reduction in MADRS score, a 53.3% response rate, and a 20% remission rate at a 12mg…
How a data lakehouse can give you a panoramic view of your AI-enabled clinical trials
In recent years, the term “data lakehouse” has entered the lexicon of data professionals. For AI-enabled clinical trials, the lakehouse architecture promises seamless integration of diverse data streams, spanning patient health records to real-time sensor data, all processed efficiently and queried in structured formats. The lakehouse architecture aims to provide a comprehensive overview of data,…
NLP in drug discovery and the quest for the ‘right’ research elements
In drug discovery and development, data sources are as diverse as they are plentiful. There are comprehensive databases brimming with molecular targets, cellular processes, genomic sequences, proteomic profiles, and metabolite patterns that shed light on disease pathways. Data possibilities in the patient care realm are similarly vast, spanning electronic medical records, imaging datasets, and even…
GLP-1 drug tirzepatide shines in SURMOUNT-3 trial with weight loss of 26.6%
In the phase 3 SURMOUNT-3 trial, tirzepatide recipients saw some of the most impressive weight loss results among trials of GLP-1 drugs, including most notably semaglutide. In the study, participants’ total mean weight loss was 26.6% over 84 weeks following a 12-week intensive lifestyle intervention and subsequent tirzepatide treatment. In all, participants who received tirzepatide…
Mirikizumab shows continued promise for ulcerative colitis after regulatory hiccup
Lilly has announced that its interleukin-23 blocker mirikizumab demonstrated promise in patients with moderately to severely active ulcerative colitis (UC) in the ongoing open-label LUCENT-3 extension study. The trial evaluated two-year efficacy and safety of the monoclonal antibody in patients who had previously undergone UC treatments, including biologics, that were ineffective, stopped working, or were…
Guselkumab shows durable benefits in Crohn’s disease in LTE of phase 2 study
The interleukin-23 blocker guselkumab (Tremfya) continues to show promise in treating Crohn’s disease (CD). First winning FDA approval for plaque psoriasis in 2017, guselkumab recently demonstrated robust efficacy and a consistent safety profile in the long-term extension of the GALAXI Phase 2 study for CD. Some 54.1% of patients receiving guselkumab achieved clinical remission by…
Alzheimer’s at an inflection point as drug and diagnostics breakthroughs emerge
Alzheimer’s disease research appears to be hitting its stride, thanks to recent therapeutic advances in drug development and the emergence of biomarkers to detect the condition. “All the pieces of the puzzle of precision medicine, which is already quite common in oncology, are now in place,” said Hartmuth Kolb, vice president, neuroscience biomarkers and R&D…
More than a century after its synthesis, MDMA could be headed for FDA approval for PTSD
First synthesized in 1912 by Merck, the empathogenic drug 3,4-Methylenedioxymethamphetamine (MDMA) is inching toward FDA approval following the positive results of a phase 3 study. The recently concluded phase 3 study, MAPP2, published in Nature Medicine, found that MDMA-assisted therapy significantly outperforms traditional talk therapy in reducing PTSD symptoms. Participants receiving MDMA-AT had an 86.5%…
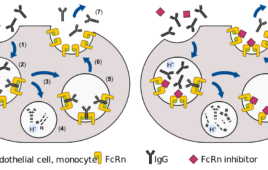
The multi-billion dollar promise of efgartigimod and the broader FcRn inhibitor market
Thanks to promising candidates such as efgartigimod, the Fc receptor (FcRn) inhibitor market is poised to reshape the autoimmune disease treatment landscape. Sales projections for the market top $10 billion, as Driehaus Capital Management estimated in 2020. The addressable U.S. patient base spans more than 228,500 individuals across various conditions including myasthenia gravis, warm autoimmune…
30 biotech startups making waves
The biotech industry is facing a reckoning in 2023. To date, roughly 100 biopharmas have cut workers this year, matching the total number of layoffs in the sector in 2022. Many biotech startups have been hit hard. The wave of job cuts comes on the heels of a biotech boom following the COVID-19 pandemic, when…
How synthetic data accelerates oncology research and drug development
Synthetic data in oncology is transforming how researchers and developers approach real-world evidence. They often need this evidence to test hypotheses, predict outcomes and develop algorithms. But privacy constraints and access related to patient data can create delays and lengthen project timelines. Oncology drug researchers and developers have recently begun using synthetic data in oncology…
At Day 21, low-dose ketamine KET01 shows no statistical edge over placebo
Ketabon GmbH revealed positive top-line results from its phase 2 KET01-02 study investigating KET01, an oral slow-release formulation of oral ketamine, for treatment-resistant depression (TRD). Results were promising initially. Investigators noted improvements in depression severity as early as day 4, but data were not statistically significant over placebo at day 21. A look at Ketabon…
A checklist for unlocking the promise of AI in clinical trials
AI algorithms offer a myriad of advantages for clinical trials. AI techniques can, for instance, support patient enrollment and site selection, improve data quality and enhance patient outcomes. AI algorithms — combined with an effective digital infrastructure — can also help aggregate and manage clinical trial data in real time, as Deloitte has noted. Last…
New AI tool InClinico predicts clinical trial outcomes with 79% accuracy: A closer look with InSilico CEO, Alex Zhavoronkov
Roughly nine out of ten clinical trials fail as a result of factors such as lack of efficacy and unmanageable toxicity, as the journal Pharma Excipients has noted. After seven years of development, Insilico Medicine has scored a breakthrough with its generative AI tool, inClinico. In particular, the tool demonstrated 79% accuracy in predicting the…
In data we trust: AI’s growing influence on drug development
The journey to developing a successful drug, theoretically, may appear linear: you discover the right drug, find the suitable patient and administer it at the right time. The reality, however, often deviates from this straightforward path. Aligning these three variables remains notoriously difficult, often leading to elongated timelines strewn with failures, sometimes extending over a…
Novel therapy shows promise in improving corneal edema treatment
Cataracts are one of the most common ailments of an aging population, manifesting in the fifth or sixth decade of life. This condition, which leads to blurred or cloudy vision, is caused by proteins that break down in the lens of our eyes, forming an increasingly opaque mass. Although cataracts can cause blindness if untreated,…
A deep dive into AWS’s strategy with generative AI and ML in life sciences
The AI market is witnessing meteoric growth, with projections hinting at a potentially staggering increase over the next decade. Against this backdrop of rapid AI evolution, we recently spoke with Tehsin Syed, general manager of AWS Health. Syed shared that Amazon Web Services (AWS) is seeing growing interest from Big Pharma firms. “Nine of the…
FDA publishes draft guidance for psychedelic drug development
In a sign of shifting attitudes, the FDA has released draft guidance to facilitate the development of psychedelic drugs. Historically, psychedelics have a long history of use in certain cultures, such as the indigenous tribes of the Amazon Basin and Native American communities, for millennia as sacraments.These substances were used not only recreationally but also…
CGT demands vision, partnership and patient-centricity to transform healthcare
As personalized medicine continues to progress, cell and gene therapy (CGT) development is poised to enable tremendous medical breakthroughs. Unlike traditional treatments which merely manage symptoms, CGT could treat the root biological cause of many diseases: the faulty genes. “The cell and gene therapy space is truly exciting. What this ultimately means for patients and…
Guselkumab could offer hope for psoriatic arthritis patients resistant to TNF inhibitors
Janssen continues to strengthen the case that its interleukin-23 inhibitor TREMFYA (guselkumab) offers promise to many patients with psoriatic arthritis (PsA). The company’s phase 3b COSMOS clinical trial involving 189 patients with active PsA and an inadequate response to one to two previous tumor necrosis factor (TNF) inhibitors, guselkumab showed sustained improvements in measures of…
8 considerations to boost clinical trial productivity with AI while dodging hallucination hurdles
The development of new drugs is undeniably a data-intensive endeavor. Despite impressive advances in AI over the past years, researchers often continue to grapple with crushing data volumes. This hurdle is particularly apparent in clinical trials, where crucial data is often stored in machine-unfriendly formats such as PDFs, PowerPoint or HTML or other formats. This article explores…
PsychoGenics’ SmartCube prompts a reevaluation of CNS drug discovery
In an era of rapid AI progress, the quest to pioneer the first AI-developed drug candidates has led to an increasing number of these drug candidates entering clinical trials. One contender is ulotaront, an antipsychotic drug, that fared well in a phase 3 schizophrenia study published in NEJM in 2020. Sunovion discovered the drug in…
A current perspective on machine learning’s role in advancing clinical trials diversity
The year 2020 was a watershed moment for many reasons, but notably, it cast a light on the pervasive health and social inequities that have long marred the U.S. The COVID-19 pandemic hit diverse populations disproportionately hard, as Deloitte and others have noted. Additionally, the tragic deaths of George Floyd, Breonna Taylor and others provoked…