The recent emergence of H5N1 avian influenza in humans and many other animals has intensified global efforts to prepare for a potential pandemic. Public health agencies and international organizations are collaborating with pharmaceutical companies and academic institutions to develop vaccines, treatments, and strategies to mitigate the impact of an outbreak. The CDC, for instance, has…
Best-selling pharmaceuticals of 2023 reveal a shift in pharma landscape
Note: This feature on the best-selling pharmaceuticals of 2023 was updated on March 27. Changes are possible as more data become available. Sales of drugs with more than one developer are added together (as in the case of the COVID-19 vaccine Comirnaty) unless one of those companies records the net sales for it, i.e., Dupixent.…
Lumen Bioscience cracks the code on spirulina as a biologics factory for c. diff, metabolic disease and more
Clostridioides difficile, commonly known as C. diff, is a significant health threat in the U.S. Recent estimates suggest that C. diff, a common bacteria, can cause infection in roughly 500,000 patients annually in the U.S., with around 30,000 of these cases resulting in death. “Actually, it’s more like 5 million when you think about it…
Vaccine mega-trials: Rare behemoths in the vaccine trial landscape
Abstract The vast majority of vaccines are prophylactic in nature. As a result, the demonstration of their efficacy paradoxically requires the infectious disease to occur among non-diseased study participants randomized between investigational vaccine and appropriate control groups. The statistics of vaccine efficacy (VE) calculation are nearly entirely and solely based on the number of observed…
Biden names 31 tech hubs: Here are 10 relevant to pharma and biotech
Traditionally, the tech and biotech sectors in the U.S. have been concentrated in a handful of regions — most notably in areas such as Boston, Seattle, Silicon Valley and Southern California. But the Biden administration aims to distribute innovation more evenly through the U.S. To that end, the administration has designated 31 tech hubs across…
An overview of the RSV vaccine landscape: GSK aims to extend its approval of Arexvy?
GSK (NYSE:GSK) is aiming to expand the label for its respiratory syncytial virus (RSV) vaccine Arexvy, which was the first to win FDA approval. The firm is now eyeing an extension of the label to include adults aged 50 to 59, bolstered by encouraging preliminary data from a phase 3 study. The recent data from…
Pfizer Ignite: Kathy Fernando’s vision for accelerating biotech innovation
Kathy Fernando, the senior vice president, head of Pfizer Ignite and Pfizer CentreOne, has had a professional trajectory marked by pivotal serendipities. One occurred when attending a seminar at the University of Pennsylvania, where she met Dr. Drew Weissman, a prominent immunologist and RNA vaccine researcher. Weissman, along with Katalin Karikó, recently received the Nobel…
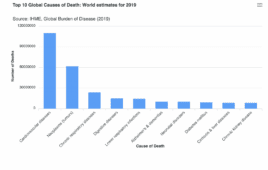
Data-driven insights into the leading causes of death, including cardiovascular disease and cancer
Data sourced from Our World in Data Cardiovascular disease and cancer remain the leading causes of death in the world, based on an analysis of data sources ranging from World Health Organization and Our World in Data. While respiratory illnesses were the third most common cause of death in 2019, deaths in this category…
Pfizer and Moderna win FDA nod for XBB.1.5 COVID-19 vaccine boosters, but projected sales pale in comparison to 2022’s
As Pfizer and Moderna receive the FDA nod for COVID-19 boosters, their 2023 global sales projections appear to be just a fraction of the previous year’s. In 2022, Pfizer and its partner BioNTech jointly sold $56 billion worth of their Comirnaty COVID-19 vaccine, marking the best-selling drug of the year. Moderna’s Spikevax COVID-19 vaccine wasn’t…
Embedding patient perspectives: Keri Yale’s impact as a change agent at Boehringer Ingelheim
When Keri Yale, the company’s head of patient affairs and engagement at Boehringer Ingelheim (BI), joined more than 25 years ago, she recognized a need to put patients at the center of the pharmaceutical industry. Since then, she has spearheaded a transformation towards greater patient centricity at the company that started with HIV/AIDS community engagement…
Top 12 reported events for Moderna and Pfizer omicron-targeting COVID-19 boosters
With updated COVID-19 boosters set to launch in the near future and COVID-related hospitalizations on the rise again, scrutiny is on the rise of emerging variants and the benefit-safety profile of COVID-19 vaccines. We performed a retrospective analysis of data from the Vaccine Adverse Event Reporting System (VAERS), focusing on the period covering the launch…
How COVID vaccine options stack up for fall 2023
[Updated September 7. Article originally posted on July 21, 2023. Updates follow in bold:] The Vaccines and Related Biological Products Advisory Committee (VRBPAC) is backing a significant shift in the current COVID-19 vaccine strategy: a move from multivalent to monovalent vaccines focusing on the XBB lineage strains. More recently, the emergence of new variants such…
Moderna and Pfizer ready updated COVID-19 boosters to combat BA.2.86 and other emerging variants
Against the backdrop of a nearly 16% spike in COVID-19 hospitalizations in late August, according to CDC data, federal authorities are gearing up to greenlight updated boosters. Moderna announced that its latest COVID vaccine is effective against this new strain. Meanwhile, Pfizer revealed positive preclinical data for its vaccine, developed in collaboration with BioNTech. In…
Five insights on COVID-19 vaccine side effects
To date, there have been more than 770 million confirmed COVID-19 cases and nearly 7 million deaths from the virus, according to the World Health Organization (WHO). Vaccines remain one of the most potent tools in blunting the severity of SARS-CoV-2 infection, and vaccine developers have distributed more than 13.5 billion vaccine doses to date.…
Legal dispute precede Pfizer’s latest FDA nod for RSV vaccine
Amid a backdrop of recent RSV vaccine approvals, GSK and Pfizer find themselves locked in a legal spat over alleged patent infringements. Both Big Pharma giants now possess the FDA’s blessing for their respective respiratory syncytial virus (RSV) vaccines. Pfizer’s recent win came with the second approval for its Abrysvo vaccine, which the company now…
Pfizer and Moderna tout preliminary data in battle against Eris SARS-CoV-2 subvariant
COVID-19 vaccine giants Pfizer and Moderna are gearing up for a battle against the Eris SARS-CoV-2 subvariant, which has rapidly emerged as the dominant strain in the U.S. Eris now is responsible for more than one in five COVID infections, based on CDC estimates from August 6 to 19. Pfizer has noted that its most…
Moderna leans on RSV vaccine, AI and a diversified pipeline to regain competitive edge
Moderna surged to prominence thanks to its rapid development of a competitive COVID-19 vaccine. Since the early days of the pandemic, however, the company has struggled to maintain its momentum. Year-to-date, its stock is down close to 40%, trading around $110 per share. Adapting to COVID-19 variants The company hopes that its development of vaccines…
Decoding the future of RNA vaccines with Aldevron’s Venkata Indurthi
From tackling the COVID-19 pandemic to paving the way for future global health challenges, RNA vaccines have rapidly gained attention in recent years. Researchers are already working to extend their capabilities for next-gen medicines. For example, scientists are working to create multivalent RNA formulations that can fight multiple virus variants. They are also exploring AI…
‘Long vax’ phenomenon gets closer look in recent studies
In 2020, researchers witnessed the emergence of post-acute sequelae of SARS-CoV-2 (PASC) — more commonly referred to as “long COVID.” Now, the notion of “long vax,” persistent and varying symptoms following COVID-19 vaccination, has come into focus, as Science has noted. This phenomenon, while not as widespread as long COVID, has concerned some in the…
AdAPT-001 oncolytic adenovirus shows promising phase 1 cancer treatment results
Oncolytic adenoviruses have won significant attention in recent years as a novel approach to cancer treatment. One example of the trend is AdAPT-001 TGF-ß Trap, an engineered variant of the common cold virus equipped with a transforming growth factor-beta (TGF-β) “trap.” This mechanism is designed to latch onto and neutralize TGF-β, an immunosuppressive cytokine involved…
Codagenix taps synthetic biology and machine learning in vaccine development
In the quest to outsmart viral foes such as SARS-CoV-2, RSV and influenza, Codagenix, a clinical-stage biotech firm based in Farmingdale, New York, has engaged a unique arsenal: the intersection of synthetic biology and machine learning. Their weapon of choice is a blend of live-attenuated virus design and codon deoptimization technology. Their process involves introducing…
Unraveling the impact of FDORA and PREVENT Pandemics Acts on the life sciences
As the world continues to grapple with global health challenges, the role of science and biotech law has taken center stage in shaping public health policy and innovation. The FDORA and PREVENT Pandemics Acts are poised to help shape the landscape. In a recent interview, life sciences attorney Barbara Binzak Blumenfeld offers insights into significant…
The 50 best-selling pharmaceuticals of 2022: COVID-19 vaccines poised to take a step back
The COVID-19 pandemic has had a profound impact on the best-selling pharmaceuticals, leading to shifts in the list with Pfizer and BioNTech’s Comirnaty surpassing AbbVie’s Humira for the No. 1 spot in 2021. That momentum continued in 2022, with Pfizer and BioNTech jointly raking in $59.1 billion in revenue from the sales of the COVID-19…
FDA authorization of vilobelimab signals new opportunities for drug developers in inflammatory diseases
The FDA has granted InflaRx (Nasdaq:IFRX) emergency use authorization (EUA) for the monoclonal antibody Gohibic (vilobelimab) to treat critically-ill COVID-19 patients. The company’s shares were up yesterday almost 84% to $3.77. Today, its shares jumped an additional 62% to $6.10. The EUA represents a significant advance for the Jena, Germany–based company, which on March 31,…
Landscape overview: Moderna and Pfizer lead the race in mRNA flu vaccines, with plans for regulatory filings in 2024-2025
mRNA technology helped propel the development of some of the most successful drugs in pharma history — notably, the Comirnaty COVID-19 from Pfizer/BioNTech generated almost $56 billion in 2022 while Moderna’s Spikevax vaccine raked in $18.4 billion. But demand for COVID-19 vaccines continues to cool. In 2023, Comirnaty brought in $15 billion between its two…