In early 2022, secretive Altos Labs stunned with a record $3 billion launch to pursue anti-aging “cellular rejuvenation,” amid rumors of Jeff Bezos’ backing. But a roundup of the top 25 private healthcare and biotech companies reveals a landscape of grand ambitions to disrupt healthcare coupled with cautionary tales. Included in the mix is scandal-plagued…
From Novartis to Pfizer: A closer look at novel cell and gene therapy pricing and reimbursement strategies
Cell and gene therapies are upending the treatment of a growing number of diseases by addressing the underlying causes of genetic disorders. Yet the high costs associated with these therapies, sometimes costing multiple millions of dollars for a single treatment, pose significant challenges for patients, payers and healthcare systems. To address this matter, a growing…
Public vs. private: Who’s leading the charge in H5N1 preparedness?
The recent emergence of H5N1 avian influenza in humans and many other animals has intensified global efforts to prepare for a potential pandemic. Public health agencies and international organizations are collaborating with pharmaceutical companies and academic institutions to develop vaccines, treatments, and strategies to mitigate the impact of an outbreak. The CDC, for instance, has…
FDA approves pair of therapies for rare pediatric cancers: Novartis’ Lutathera and Day One’s Ojemda
The FDA has signed off on two novel therapies targeting rare pediatric cancers. Novartis’ Lutathera targets aggressive gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in children 12 and up, while Day One Biopharma’s Ojemda (tovorafenib) tackles treatment-resistant BRAF-mutated relapsed or refractory pediatric low-grade glioma (pLGG) with a BRAF fusion or rearrangement, or BRAF V600 mutation These approvals offer…
Medincell, AbbVie partner on long-term injectable therapies
Medincell and AbbVie today announced a collaboration to co-develop and commercialize up to six therapeutic products across several areas. The partnership spans multiple therapeutic spaces and indications. Under the agreement, Medincell will use its commercial-stage, long-acting injectable technology platform to formulate innovative therapies. It plans to conduct formulation activities and preclinical studies, including supportive CMC…
The rise of ‘Ozempic babies’ and the uncharted territory of semaglutide in pregnancy
Ozempic, Rybelsus and Wegovy have transformed the diabetes and weight loss treatment landscape, but when it comes to the impact of their active ingredient, semaglutide, on fetal development, “the answer is we do not know,” said Dr. Marijane Hynes, clinical professor of medicine at the George Washington University School of Medicine and Health Sciences. Hynes…
Global biotech VC trends in Q1 2024
The first quarter of 2024 saw a flurry of funding activity in the biotech sector, with early-to-mid-stage companies attracting most of the investment dollars. Among the most notable late-stage deals was blood-based cancer diagnostics firm Freenome‘s $254 million Series E round. The South San Francisco-based company, which is developing a platform for early cancer detection…
Machine learning model forecasts Lilly’s Mounjaro sales to triple in 2024
Novo Nordisk has received a considerable amount of attention for its meteoric growth of late, but Eli Lilly isn’t far off in that domain as of late. Lilly recently topped the S&P 500 healthcare index in terms of growth, jumping almost 34% as demand for its metabolic therapies surge. Last year, Lilly projected it would…
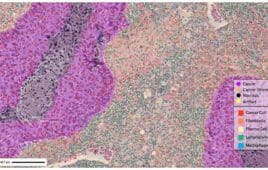
How digital pathology can help drug developers address the forked road in oncology and beyond
Digital pathology is disrupting the drug development process, replacing traditional microscopy rooted in Victorian Era approaches with high-resolution images and AI-powered analysis tools. In a recent editorial webinar, industry experts Nathan Buchbinder (chief strategy officer at Proscia), Dr. John Cochran (chief medical officer and chief pathologist at Q² Solutions, an IQVIA subsidiary), and Dr. Monika…
Pharmaceutical firms betting big on AI struggle to find the right people
Three-quarters of IT decision-makers at pharmaceutical companies plan to invest from $500,000 to $5 million in AI this year which, for many, doubles their budgets over the past year. That’s one of the findings based on the cross-industry research report involving 1,400 IT decision makers from Rackspace and AWS. “In terms of AI adoption, almost…
Why Atomwise sees a ‘generational shift’ in drug discovery with AI applied to screening
Atomwise’s AIMS (Artificial Intelligence Molecule Screening) project has demonstrated significant potential to accelerate drug discovery. In a landmark multiyear study published in Nature Scientific Reports, the project used Atomwise’s AI platform, AtomNet, to successfully identify structurally novel compounds across a diverse range of therapeutic targets. In the virtual high-throughput screening (HTS) study, AtomNet analyzed 318…
Forget efficiency, focus on shifting the standard of care with AI in drug discovery
Much has been made about AI’s potential to accelerate drug development timelines while chipping away at its often multi-billion-dollar price tag. But Abraham Heifets, CEO of Atomwise, believes that focusing on efficiency is not necessarily the right measure — or the right conversation — to have. Time for a different conversation “The two ways to…
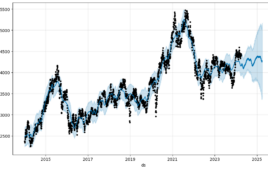
Biotech market projection for 2024 could point to more sector stability
The biotech sector could see a period of relative stability, according to a time-series analysis of the NASDAQ Biotech Index. In recent years, however, performance of the NBI has had little bearing on the rate of layoffs in the pharma and biotech sectors. A statistical analysis of the relationship between the NBI performance and the…
Clinical trials have a diversity Problem. Real-world data could be part of the solution
While diversity in clinical trials has won attention in recent years, uneven representation of diverse populations in clinical trials remains a core barrier to global healthcare equity. Real-world data (RWD) paired with artificial intelligence techniques can almost instantly analyze how well drugs work in diverse subpopulations once they hit the market. RWD can also help…
Can payers afford the new era of GLP-1 drugs? Or can they afford not to?
The latest crop of glucagon-like peptide-1 (GLP-1) agonists, such as semaglutide and tirzepatide (technically, a dual GIP/GLP-1 receptor agonist), have upended the way we treat metabolic disorders, including obesity. These drugs mimic gut hormones, improving blood sugar control and often leading to significant weight loss. But as Kyasha Sri Ranjan, Ph.D., engagement manager at Lifescience…
Inside the AI-powered Roche-PathAI companion diagnostics collaboration
PathAI and Roche Tissue Diagnostics (RTD) have inked an exclusive collaboration to develop AI-enabled companion diagnostics that builds on their initial partnership announced in October 2021. To date, PathAI and Roche have commercially launched four algorithms through the partnership. The new partnership will provide biopharma sponsors with integrated technology for developing companion diagnostics incorporating AI-based…
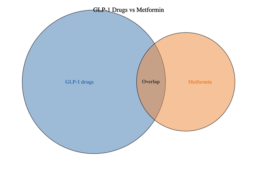
GLP-1s overtake metformin in metabolic clinical trials by a wide margin: A visual exploration
A recent review of more than 2,000 studies related at least indirectly to obesity on clinicaltrials.gov highlights the pronounced significant shift in research focus toward GLP-1 receptor agonists for a range of indications with at least some involvement of metabolic disorders. The site, which provides a robust but not-exhaustive snapshot of clinical trial activity, cites…
Unlearn CEO: Digital twins could slash clinical trial patient enrollment by 25% or more
The startup Unlearn embodies several trendy AI characteristics. Generative AI company? Check. San Francisco headquarters? Check. Aims to disrupt drug development (specifically, clinical trials) with AI? Check. Prominent AI leadership? Check. Mira Murati, the high-profile CTO of OpenAI, joined Unlearn’s board of directors in 2023. But the company’s pedigree is unique. The firm was founded…
Best-selling pharmaceuticals of 2023 reveal a shift in pharma landscape
Note: This feature on the best-selling pharmaceuticals of 2023 was updated on March 27. Changes are possible as more data become available. Sales of drugs with more than one developer are added together (as in the case of the COVID-19 vaccine Comirnaty) unless one of those companies records the net sales for it, i.e., Dupixent.…
From AI transformers to computer-based reasoning to rethinking drug design: AI pioneers discuss the future
In a packed panel discussion at GTC, moderated by NVIDIA Founder and CEO Jensen Huang, the architects of the groundbreaking transformer model gathered to explore their creation’s potential. The panel featured seven of the eight authors of the seminal “Attention Is All You Need Paper” paper, which introduced transformers — a type of neural network…
Creating chemical diversity with flow chemistry
Flow chemistry techniques are increasingly being used in drug discovery to provide cost-effective access to a wide range of structurally diverse small molecule analogs, as well as access to previously underused or inaccessible chemistries. There are several ways that this powerful technique can be used to increase structural diversity when building candidate molecules, including linear…
NVIDIA exec on how ‘NIMs’ can help biopharma navigate the challenges of deploying generative AI
The buzz surrounding generative AI may be undeniable, but its real-world impact on heavily-regulated sectors like drug discovery continues to evolve. Consequently, most drug candidates, circa 90%, continue to fail. Kimberly Powell, vice president of Healthcare at NVIDIA, believes that a new microservices-based offering known as NIMs (NVIDIA Inference Microservices) could help pharma firms navigate…
Denmark teams up with Novo Nordisk Foundation, NVIDIA to launch visionary AI research center
A collaboration between the Novo Nordisk Foundation, the Export and Investment Fund of Denmark (EIFO), and NVIDIA will establish a national AI Innovation Centre in Denmark focused on accelerating research and innovation in fields including healthcare, life science, and quantum computing. The initiative is led on the Danish side by the Novo Nordisk Foundation, which…
Iambic Therapeutics and NVIDIA partner to slash cancer drug development timelines
Using generative AI in drug discovery, Iambic Therapeutics (formerly Entos) has advanced its IAM1363 drug candidate from program launch to clinical studies in fewer than 24 months — a process that often takes several years. Iambic Therapeutics’ AI drug development milestone relied on an alliance with NVIDIA researchers and engineers and through the use of…
Q&A: How Insilico Medicine’s AI identified a new IPF drug target in record time
Idiopathic Pulmonary Fibrosis (IPF), a devastating lung disease affecting millions with increasing incidence, may have a new treatment hope thanks to a novel inhibitor of TNIK, a kinase newly implicated in fibrosis, identified using generative AI drug discovery platforms in just 18 months. Researchers at Insilico Medicine, along with international collaborators, harnessed the power of…









![[Adobe FIrefly]](https://www.drugdiscoverytrends.com/wp-content/uploads/2024/04/Firefly-Visualize-a-photo-realistic-HD-pharmaceutical-laboratory-image-with-concentrated-workers-in_-e1712103436381-268x170.png)