NVIDIA is putting the power of generative AI for drug discovery into the hands of more pharmaceutical and biotech companies with an expanded collection of AI models and flexible deployment options. More than 100 firms are already using the company’s biomolecular BioNeMo platform to accelerate the development of therapeutics. “For the first time in history,…
Iambic Therapeutics and NVIDIA partner to slash cancer drug development timelines
Using generative AI in drug discovery, Iambic Therapeutics (formerly Entos) has advanced its IAM1363 drug candidate from program launch to clinical studies in fewer than 24 months — a process that often takes several years. Iambic Therapeutics’ AI drug development milestone relied on an alliance with NVIDIA researchers and engineers and through the use of…
Could LSD change the game in anxiety treatment?
A once-controversial psychedelic substance could potentially be a promising treatment for generalized anxiety disorder (GAD). That’s the view of Dr. Rakesh Jain, a psychiatrist with extensive experience in clinical practice, research, and education, affiliated with Texas Tech University School of Medicine. Jain expressed optimism in LSD-based therapy while acknowledging the challenges inherent in such a…
Rare diseases, immense needs: J&J’s mission to change the landscape
Rare diseases may seem niche, but their impact is far from small. An estimated 7,000 rare diseases exist, collectively affecting a 300 million people worldwide. This immense burden of disease, coupled with a profound lack of treatment options, underscores the urgent need for innovation. “Actually, the total burden of disease and unmet medical need [for…
Big Pharma shakeup: This chart reveals the new top dog of 2023
In the Aesopian fable, the tortoise’s steady focus and persistence overcomes the hare’s bursts of speed. 2023 saw a similar shift in the pharma sector. While Pfizer, thanks to blockbuster COVID vaccine sales, rocketed to unprecedented heights in 2022 surpassing $100 billion in revenue, its fortunes reversed in 2023. As COVID product demand plummeted, Pfizer’s…
Survey: Wielding AI magic in clinical trials requires a master’s touch
eClinical’s Industry Outlook 2024 report highlights a significant acceleration in AI/ML adoption for clinical trials. Over half of professionals (53%) in functions like clinical operations, data management, and biometrics now see these technologies as central to streamlining trials by 2024, surpassing the emphasis on automation that dominated last year. Despite this hype cycle, a core…
What’s next for biotech? Q4 2023 funding trends point beyond the usual suspects
Oncology may continue be one of the hottest sectors across the pharma sector, but other therapeutic areas are catching up in terms of innovation and investment. While oncology and hematology jointly accounted for about one-third of the new FDA approvals in 2023, investors are increasingly betting on precision medicine, advanced drug delivery systems and the use of…
AbbVie bets on quality of life approach to gain share in migraine treatment market
In a crowded migraine treatment landscape, AbbVie is aiming to differentiate itself by redefining migraine treatment beyond just counting headache days and other traditional clinical endpoints. At the European Headache Congress (EHC), the company touted its patient-centered approach in evaluating quality of life, daily functioning, rapid relief, and balanced benefit-risk profiles, while aiming to continue…
Two-thirds of pharma companies plan to up IT investments in 2024, survey finds
Two out of three pharma companies (67%) plan to ramp up investment in IT, including AI, over the next 12 months, according to a survey from the cloud vendor Rackspace Technology and Dell/VMware conducted in October 2023. About the same amount, 68%, reported challenges in recruiting and hiring talent skilled in cloud and AI technology.…
Psilocybin analog leads to 79% of remission in mid-stage depression trial
After only two doses, nearly eight out of ten participants in a phase 2 trial experienced remission from major depressive disorder (MDD) at six weeks, highlighting the potential of Cybin’s deuterated psilocybin analog, CYB003. In 2021, an estimated 21.0 million adults in the United States had at least one major depressive episode, representing 8.3% of…
Digital dreams and realities clash pharma and biotech in 2023
The tale of digital pharma and biotech in 2023 is one of two realities. In one corner, you have AI and digital-focused startups and sometimes executives at Big Pharma companies with grand AI ambitions proclaiming the power of the technology. But on the other side of the pharma-AI coin is a more cautious crowd. Here,…
GLP-1 drug tirzepatide shines in SURMOUNT-3 trial with weight loss of 26.6%
In the phase 3 SURMOUNT-3 trial, tirzepatide recipients saw some of the most impressive weight loss results among trials of GLP-1 drugs, including most notably semaglutide. In the study, participants’ total mean weight loss was 26.6% over 84 weeks following a 12-week intensive lifestyle intervention and subsequent tirzepatide treatment. In all, participants who received tirzepatide…
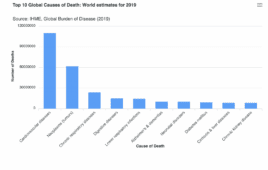
Data-driven insights into the leading causes of death, including cardiovascular disease and cancer
Data sourced from Our World in Data Cardiovascular disease and cancer remain the leading causes of death in the world, based on an analysis of data sources ranging from World Health Organization and Our World in Data. While respiratory illnesses were the third most common cause of death in 2019, deaths in this category…
Gate Neurosciences takes on depression with event-driven pharmacology
Much of the world is grappling with a mental health crisis — with soaring rates of depression and anxiety. Last year, the startup Gate Neurosciences emerged with a novel approach. While the Carmel, Indiana–based company is focused on synaptic plasticity like many other contemporary CNS companies, it diverges in its strategy. Rather than using a…
eClinical Solutions Q&A: The quest to transform raw data into drug discovery gold
Top pharmaceutical companies sponsor over a hundred clinical trials annually, generating vast amounts of data. Harnessing this deluge is a monumental task. eClinical Solutions, led by CEO Raj Indupuri, tackles this through advanced applications of data analytics and machine learning with an emphasis on AI in clinical trials optimization. Specifically, eClinical Solutions taps AI/ML for…
A new microparticle approach may offer hope for reversing multiple sclerosis
Johns Hopkins researchers have made strides in a study focusing on multiple sclerosis. By applying microparticles to activate regulatory T cells, they were able to reverse MS-like symptoms in mice. There is no cure for multiple sclerosis (MS). But a recent study by Johns Hopkins Medicine shows encouraging progress towards: They have demonstrated the ability…
Speed up regulatory submissions: A guide for efficiency
Drug development involves many rounds of regulatory approval and oversight, including multiple stages of regulatory submissions. In the U.S., these include the Investigational New Drug Application (IND) before clinical trials in humans, the New Drug Application (NDA) or Biologics License Application (BLA), prior to commercial sale and Annual Product Quality Reviews (APQR) to monitor ongoing…
Behind the scenes: Dr. Andy Beck, PathAI CEO, talks PathExplore
In a recent conversation with Dr. Andy Beck, co-founder and CEO of PathAI, we had the opportunity to discuss PathExplore, an AI-driven platform that aims to transform the way tumor microenvironment (TME) analysis is conducted. Traditional methods such as manual pathology, multiplex immunofluorescence and single-cell omics often face limitations, including high costs or tissue consumption.…
Nvidia launches BioNeMo Cloud as a breakthrough AI service for drug discovery research
During Nvidia’s (Nasdaq:NVDA) GTC event, the company introduced BioNeMo Cloud, a new component to their AI Foundations suite. This service, designed to streamline life sciences research, drug discovery and protein engineering, provides researchers with access to pretrained AI models, allowing for customization with proprietary data. Offered as a cloud service, BioNeMo Cloud enables accelerated drug…
Psychedelic research firm Lucy Scientific Discovery misses IPO target
Lucy Scientific Discovery Inc. (Nasdaq:LSDI), a Canadian firm that focuses on developing and manufacturing psychotropics-based medicines, had its initial public offering (IPO) today. The company had priced 1,875,000 common shares at a public offering price of $4.00 per share. On its first day of trading, however, the stock dropped by 25.25%, reaching a closing price…
Neuron23 launches clinical trial for NEU-723 in Parkinson’s with companion diagnostic in development
South San Francisco–based Neuron23 has started the first-in-human Phase 1 trial of NEU-723 for the treatment of Parkinson’s disease. NEU-723 is a highly potent and selective leucine-rich repeat kinase 2 (LRRK2) inhibitor. This clinical trial will evaluate the safety, tolerability and pharmacokinetics of NEU-723 in healthy volunteers. A targeted approach The company has also collaborated…
How Parallel Bio aims to turn drug development on its head
In 2021, two scientists founded Parallel Bio to harness the human body’s ability to fight disease using the immune system. The Cambridge, Massachusetts–based company is developing a platform for drug discovery and development that replicates human immune systems at population scale. The company has grand ambitions. “Nearly 95% of drugs that make it to human trials fail…
Guselkumab analysis shows psoriatic arthritis patients with early efficacy had sustained benefit
At the American College of Rheumatology (ACR) meeting, Janssen Immunology presented a Tremfya (guselkumab) posthoc analysis demonstrating stringent disease activity control at two years in patients with psoriatic arthritis. In addition, the analysis of Phase 3 DISCOVER-2 data indicated that patients with active psoriatic arthritis with a response at week eight to the IL-23 inhibitor…
Pfizer and BioNTech’s updated bivalent booster retains protection against new omicron sublineages
Pfizer (NYSE:PFE) and BioNTech (Nasdaq:BNTX) have announced that their omicron BA.4/BA.5-adapted bivalent booster fared well against novel omicron variants in neutralizing antibody detection tests. The company published the results from the bivalent booster tests against the BA.4.6, BA.2.75.2, BQ.1.1 and XBB.1 subvariants on the preprint server BioRxiv. One month after administering a fourth vaccine dose, the…
Novartis mulls spinning off ophthalmology and respiratory divisions
As part of its ongoing restructuring efforts, Novartis AG (NYSE:NVS) is reportedly considering selling its ophthalmology and respiratory businesses, according to Bloomberg. Private equity firms are reportedly sizing up the business units. According to the company’s website, its core therapeutic areas are cardiovascular, hematology, solid tumors, immunology and neuroscience. Novartis’s ophthalmology division focuses on various therapies…