Endometriosis, a condition where endometrial tissue grows outside the uterus, has a strong genetic underpinning. A new study published in the Journal of Molecular Diagnostics sheds light on this connection. Researchers from a team including Genzeva, LumaGene, RYLTI Biopharma, Brigham & Women’s Hospital of Harvard University and QIAGEN Digital Insights used a unique approach in…
Unleashing a new frontier: The power of germline clinico-genomic data to drive therapeutic development
Over the past decade, the use of deeper sources of real-world data across all stages of the drug development life cycle has become increasingly important to guide disease understanding, trial designs, clinical guidelines, regulatory submissions and post-market studies. The advent of these deeper sources was prompted by the HITECH Act, which had the effect of…
NVIDIA expands BioNeMo platform with new foundation models and microservices for AI-powered Drug Discovery
NVIDIA is putting the power of generative AI for drug discovery into the hands of more pharmaceutical and biotech companies with an expanded collection of AI models and flexible deployment options. More than 100 firms are already using the company’s biomolecular BioNeMo platform to accelerate the development of therapeutics. “For the first time in history,…
Navigating the cancer progression pathway with liquid biopsy
Tumor heterogeneity analysis is a challenging but critical step for the advancement of cancer therapy research. DNA mutation interrogation and gene expression pattern evaluation are crucial for predicting a tumor’s response or resistance to specific drug or hormone treatments. Liquid biopsy has emerged as a promising tool, offering a rapid, minimally invasive method for early…
Microsoft and 1910 Genetics: AI-powered partnership targets billion-dollar savings and growth in drug discovery
The pharmaceutical industry is at a critical juncture: AI and other technological advances offer unprecedented potential, yet the cost of developing new drugs has ballooned for decades, surpassing $2 billion in recent years with the projected return on investment (ROI) falling to a mere 1.2% in 2022, according to Deloitte. Another dimension of the problem…
Culmination Bio partners with Merck on disease-agnostic patient data
The bioinformatics startup Culmination Bio had received financial backing from Merck’s venture arm. Now, the Utah-based company is collaborating directly with the pharmaceutical giant on an autoimmune disease research project. According to Culmination Bio‘s CEO Dr. Lincoln Nadauld, the collaboration highlights the value of the startup’s ability to provide rapid access to high-quality longitudinal patient…
Could an ‘extreme’ hibernating ground squirrel unlock new obesity treatments?
In late 2023, Eli Lilly, whose stock is now up close to 80% over the past year, inked a deal with the Emeryville, California–based Fauna Bio potentially worth $494 million that focuses on the discovery of novel drug targets for treating obesity. In 2020, Fauna entered into an obesity-focused collaboration with Novo Nordisk, Lilly’s primary…
Recursion partners with NVIDIA to unveil Phenom-Beta, democratizing access to its $1 Billion phenomics investment
In sync with the JP Morgan Health Care Conference, Recursion Pharmaceuticals has unveiled Phenom-Beta, a deep learning model designed to transform cell microscopy images into meaningful biological representations. “Phenom-Beta allows you to take images of human cells from a microscope or other sources and turn them into mathematical representations of biology,” said Chris Gibson, CEO…
Using AI to unlock new uses for existing cancer medicines
Repurposing is a drug development strategy that has been widely applied in cancer. This strategy, sometimes called label expansion, involves obtaining FDA approval to market a drug for the treatment of new indications, alone or in combination with other drugs. Not only can this approach extend the window of patent protection for a commercialized drug,…
Nvidia-Genentech AI drug discovery alliance unites computing brawn with biological brains
Technically, graphics processing and AI hardware powerhouse Nvidia is also a drug discovery company. It may not discover drugs in-house, but it has developed BioNeMo, a comprehensive generative AI platform for drug discovery and Clara, a collection of healthcare frameworks, applications, and tools, including for biopharma. Nvidia partners include Amgen, AstraZeneca, GSK and Insilico Medicine.…
Unlocking the secrets of cellular immunogenicity: A deep dive in ELISpot assays
Next generation therapies and vaccine research have recently dominated headlines and scientific discovery. This research and its ability to treat previously untreatable conditions has rightfully excited the scientific and medical communities worldwide. But the complexities involved require a nuanced approach to ensure the treatment is safe and effective. Integrating cell culture into cellular immunity testing…
How Lantern Pharma and Code Ocean partnered on oncology drug development
A vision for data-driven drug development in oncology When Peter Carr, principal software architect of Lantern Pharma, stepped into his full-time role in September 2020, the company was on the cusp of a transformation. While AI had been a focus for a number of years, a fresh infusion of cash provided a possibility of expanding…
AI in antibody drug discovery as a tool, not a magic wand
AI in drug discovery is a topic that gets an outsized amount of attention, observes Carl Hansen, CEO of AbCellera, a company specializing in antibody drug discovery. “It’s as if people are saying, ‘AI is here, it’s going to save us. Thank God, we’re finally gonna be able to create drugs,” he said. “To me,…
10 rising stars of North American biotech: Cities emerging as life sciences hubs
As the biotech industry matures, several North American cities are emerging biotech hubs, aiming to become the next epicenters of innovation and growth. While traditional hubs like Boston and San Francisco continue to dominate, a new wave of cities is making strides in biotech. For instance, Pittsburgh, an up-and-coming biotech hub, is tapping its biomanufacturing…
Ginkgo Bioworks and Google Cloud forge five-year AI and biology partnership
Founded in 2008, Ginkgo Bioworks’ stock jumped almost 25% on August 29, hitting $2.22, after unveiling a five-year partnership with Google Cloud. The partnership centers around the development of novel AI tools for biology and biosecurity. In particular, Ginkgo hopes to further its mission to make biology easier to engineer in the AI era. Opting…
Mapping the cancer patient journey with liquid biopsy
According to the American Cancer Society, one in two men and one in three women will be diagnosed with cancer at some point in their lives. Patients seek treatment to shrink their tumors and ideally achieve remission; however, there is no one-size-fits-all approach. At its core, cancer is a genetic disease: Different types of cancer that affect…
30 biotech startups making waves
The biotech industry is facing a reckoning in 2023. To date, roughly 100 biopharmas have cut workers this year, matching the total number of layoffs in the sector in 2022. Many biotech startups have been hit hard. The wave of job cuts comes on the heels of a biotech boom following the COVID-19 pandemic, when…
10x Genomics’ Chromium platform sheds light on CAR T-cell therapy persistence
Therapy persistence is a vital factor in determining the success of CAR-Ts for blood cancers like leukemia. While CAR-Ts hold great promise for blood cancers such as leukemia, in some cases, the durability of the treatment falls short, leading to a potential relapse. A positive study from researchers at University College London, Great Ormond Street…
Decoding the future of RNA vaccines with Aldevron’s Venkata Indurthi
From tackling the COVID-19 pandemic to paving the way for future global health challenges, RNA vaccines have rapidly gained attention in recent years. Researchers are already working to extend their capabilities for next-gen medicines. For example, scientists are working to create multivalent RNA formulations that can fight multiple virus variants. They are also exploring AI…
Illumina’s record EU fine could drive smaller M&A pharma deals
Illumina, the world’s largest gene sequencing company, faces a record fine from the E.U. for acquiring startup Grail without approval. The $8 billion deal closed in August 2021 despite ongoing EU. and U.S. investigations. A year later, the EU prohibited the merger, citing reduced innovation and choice. The expected $453 million fine, which would be…
SandboxAQ, bearing Alphabet’s DNA, eyes quantum-inspired breakthroughs in drug discovery
Emerging as a spinoff from Alphabet’s experimental division X, SandboxAQ recently pulled back the curtain on its latest endeavor — the biopharma molecular simulation division AQBioSim. The venture has attracted investments from significant figures like former Alphabet CEO Eric Schmidt, who is now the chair of Sandbox AQ, and Salesforce.com Inc. founder Marc Benioff’s Time…
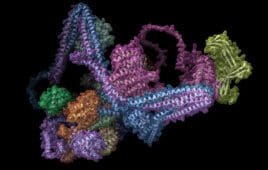
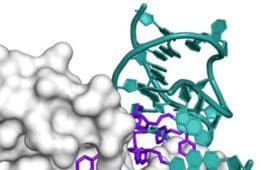
Decoding the enigma of the commander complex: Employing AlphaFold2 to illuminate biological structures
Machine learning algorithms, such as Alphabet’s neural network-based model AlphaFold2, are steadily transforming medical research, shedding light on complex biological structures. A recent case in point involves research using the technology to explore the Commander complex, a 16-protein complex crucial for cellular protein transport processes. This complex is not only vital for normal cellular function,…
Proteomics can help us realize a future of precision healthcare: Takeaways from a 2023 prediction
Clinicians are ready and eager for the future of diagnostic and prognostic models — ones that are built on proteomics. I have been feeling and hearing one key theme in 2022 – physicians are ready, even dare I say eager, to get their hands on proteomic tests and tools that better empower and equip them…
The top 15 drug discovery and development predictions of 2023
Technology enabling the discovery and development of new drugs is rapidly evolving. We asked representatives from KBI Biopharma, Phylloceuticals and Provectus Algae for their predictions on the platforms and technologies that will have the biggest impact on drug discovery efforts in 2023. 1. 2023 could be ‘massive showcase’ for ML in pharma Drug developers are…
AWS launches Amazon Omics to sift through multiomic data
At AWS Re:Invent in Las Vegas, Amazon (Nasdaq:AMZN) subsidiary AWS launched Amazon Omics to help researchers sift through genomic, transcriptomic and proteomic data. The volume of such data is exploding. The National Human Genome Research Institute estimates that researchers will need approximately 40 exabytes to store genome-sequence data generated internationally by 2025. One exabyte equates to one…