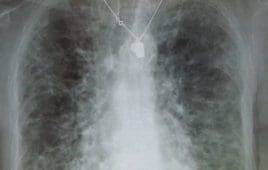
Idiopathic Pulmonary Fibrosis (IPF), a devastating lung disease affecting millions with increasing incidence, may have a new treatment hope thanks to a novel inhibitor of TNIK, a kinase newly implicated in fibrosis, identified using generative AI drug discovery platforms in just 18 months. Researchers at Insilico Medicine, along with international collaborators, harnessed the power of…
Insilico’s AI-discovered INS018_055 graduates to phase 2
Roughly a year ago, Insilico Medicine announced that it had dosed the first patient in a phase 1 study of INS018_055, an AI-discovered, first-in-class small molecule inhibitor. Now, the company has progressed to the next stage, launching a phase 2 study for the drug candidate. Insilico, a founding member of NVIDIA Inception, developed its AI…
Why Aria Pharmaceuticals is upbeat about two novel IPF treatment candidates
Small molecule drug developer Aria Pharmaceuticals (Palo Alto, Calif.) recently revealed that two investigational treatments for idiopathic pulmonary fibrosis (IPF), TXR-1002 and TXR-1007, demonstrated promising results in preclinical research. There are currently only two FDA-approved treatments for IPF on the market — nintedanib (Ofev) from Boehringer Ingelheim and pirfenidone (Esbriet) from Roche. While those introductions…
Why pulmonary fibrosis deserves more attention
This September is Pulmonary Fibrosis Awareness Month, which the Pulmonary Fibrosis Foundation (PFF) and allies have launched to educate the public about the disease family. Involving scarring of the lungs, pulmonary fibrosis gradually robs the breath from patients it affects. Some 250,000 Americans live with idiopathic pulmonary fibrosis, according to PFF. At present, two drugs…
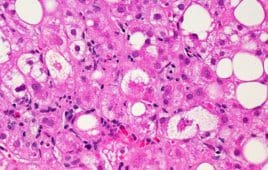
The promise of mitochondria-based therapeutics for NASH
Although nonalcoholic steatohepatitis (NASH) is one of the most common types of chronic liver disease in the U.S., it is a disease that is unfamiliar to many Americans. Despite this, its prevalence is increasing, and by some estimates, 27 million Americans will be living with NASH by 2030. NASH is a progressive condition that starts…
Aria Pharmaceuticals announces positive in vivo data for two idiopathic pulmonary fibrosis candidates
Small molecule drug developer Aria Pharmaceuticals (Palo Alto, Calif.) has announced that two investigational treatments for idiopathic pulmonary fibrosis (IPF), TXR-1002 and TXR-1007, demonstrated efficacy and tolerability in preclinical research. The two drug candidates reduced fibrosis and lung collagen staining. The company used Boehringer Ingelheim’s nintedanib as a control in the research. TXR-1002 and TXR-1007…
The pulmonary fibrosis treatment landscape: An interview with an expert
In 2014, FDA approved two medications for idiopathic pulmonary fibrosis (IPF) — nintedanib from Boehringer Ingelheim and pirfenidone from Roche (OTCMKTS:RHHBY). “That was a huge success for our community but certainly, it’s the beginning of what we need to be doing for our patients,” said Dr. Joyce Lee, a senior medical advisor for the Pulmonary…
7 notable pulmonary fibrosis research efforts
Idiopathic pulmonary fibrosis (IPF) is frequently a debilitating disease associated with significant morbidity and mortality. Although a rare disease, its incidence has increased in recent decades, and it leads to more deaths than some cancers. The treatment landscape for the condition changed in 2014 when FDA approved the first drugs indicated for IPF, nintedanib from Boehringer…