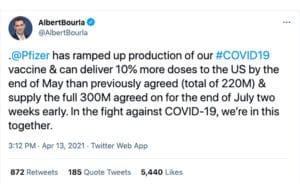
Pfizer will deliver 10% more of its COVID-19 vaccine to the U.S. by the end of May than originally planned, its CEO tweeted yesterday.
Pfizer will deliver 10% more of its COVID-19 vaccine to the U.S. by the end of May than originally planned, its CEO tweeted yesterday.
The tweet follows the federal government’s decision to recommend a temporary halt in the distribution of Johnson & Johnson’s single-dose vaccine, which has been tied to rare instances of blood clot formation in women.
Pfizer will deliver 220 million doses of its two-dose vaccine by the end of May and supply the full 300 million it agreed to for the end of July two weeks early, CEO Albert Bourla tweeted. “In the fight against COVID-19, we’re in this together,” he added.
 As of Monday, more than 6.8 million doses of the J&J single-dose vaccine had been administered in the U.S. Among recipients of those doses, six cases of a rare and severe type of blood clot have been reported. The CDC and FDA are reviewing data involving these cases.
As of Monday, more than 6.8 million doses of the J&J single-dose vaccine had been administered in the U.S. Among recipients of those doses, six cases of a rare and severe type of blood clot have been reported. The CDC and FDA are reviewing data involving these cases.
Some experts posit that the adenovirus used in J&J’s vaccine may be the culprit behind the formation of a blood clot known as cerebral venous sinus thrombosis (CVST), which federal officials said yesterday was seen in combination with low levels of blood platelets (thrombocytopenia). All six cases were in women between ages 18 and 48. One woman in Virginia has died and another in Nebraska is in critical condition, according to a report by the New York Times.
The CDC is set to convene a meeting of the Advisory Committee on Immunization Practices (ACIP) today to review the cases and assess the potential significance, while the FDA plans to review the analysis as part of its own investigation. Until the reviews conclude, the agencies recommended a pause “out of an abundance of caution,” in part to ensure healthcare providers are aware of the potential for these blood clots and can prepare for the proper recognition and management of such events.
Pfizer has already delivered nearly 123.5 million doses to the U.S., according to the CDC. Of those, 99.5 million have been administered. Moderna has delivered 104.4 million, of which 85.4 million have been administered.
By comparison, J&J has delivered 17.4 million doses of its vaccine to the U.S., of which 7.3 million have been administered, the CDC noted.
Filed Under: Drug Delivery, Drug Discovery and Development