Ypsomed announced today that it entered into a partnership with ten23 health to develop therapeutics for the YpsoDose wearable injector. Burgdorf, Switzerland-based Ypsomed designed YpsoDose for the subcutaneous self-injection of large-volume doses. ten23 health, a Swiss CDMO, offers drug development, filling and device assembly expertise to contribute to the product offering. The strategic collaboration sees…
Click chemistry breakthroughs drive Shasqi and J&J cancer alliance
In June 2023, the click chemistry-focused startup Shasqi revealed a research pact with Johnson & Johnson Enterprise Innovation. More recently, the company announced that it had expanded the research alliance, furthering work on its intratumorally injected biopolymer, known as SQL70. The collaboration will also apply its clinically validated Click Activated Protodrugs Against Cancer (CAPAC) technology…
Nobel-connected startup Shasqi deepens J&J partnership on CAPAC platform
San Francisco-based oncology startup, Shasqi, announced an expansion of its research collaboration with Johnson & Johnson Enterprise Innovation. The partnership centers on Shasqi‘s CAPAC platform, which is an abbreviation for Click-Activated Protodrugs Against Cancer. The platform separates tumor-targeting from the actual drug payload with the aim of maximizing potency while minimizing toxic side effects. Shasqi’s…
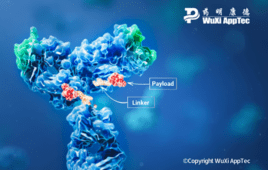
Exploring future cancer therapies: Designing linkers to increase ADC efficacy and reduce toxicity
Antibody-drug conjugates (ADCs) represent a significant paradigm shift in cancer treatment, marrying the precise target recognition of monoclonal antibodies with the potent cell-killing capabilities of cytotoxic agents. In contrast to traditional small-molecule therapies, ADCs are multifaceted structures with three distinct components — i.e., antibody, cytotoxic payload and a linker — each playing a crucial role…
Assessing the techbio landscape: hype or substance?
The venture capital firm Artis Ventures, founded in 2001, coined the term “techbio” sector to describe biotech platforms where technology and engineering take the lead in advancing drug discovery and biomanufacturing. In circa 2019, the firm contributed to shaping the techbio landscape by setting up venture capital fund named Artis techbio that bridges the gap…
This biodegradable brain implant delivers cancer-treating drugs
Researchers say they developed a biodegradable brain implant capable of helping to deliver chemotherapy drugs directly to tumors. Medscape News reported that the research marks another step toward using ultrasound to combat cancer. According to the team, led by Thanh Nguyen, these drugs can penetrate the blood-brain barrier to reach these brain tumors. Nguyen serves…
A new microparticle approach may offer hope for reversing multiple sclerosis
Johns Hopkins researchers have made strides in a study focusing on multiple sclerosis. By applying microparticles to activate regulatory T cells, they were able to reverse MS-like symptoms in mice. There is no cure for multiple sclerosis (MS). But a recent study by Johns Hopkins Medicine shows encouraging progress towards: They have demonstrated the ability…
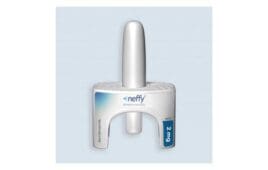
FDA accepts NDA for intranasal epinephrine from ARS Pharmaceuticals
ARS Pharmaceuticals announced today that the FDA accepted its new drug application (NDA) for neffy, its intranasal epinephrine. The offering covers the emergency treatment of severe type I allergic reactions in children and adults weighing 30 kg (66 pounds) or more. The company set an anticipated target action date of mid-2023 for its PDUFA (Prescription…
Johnson & Johnson is bringing the world’s first drug-eluting contact lens to market
Johnson & Johnson has a potential alternative to eye drops with its drug-eluting Acuvue Theravision contact lens. As far back as the early 1960s, researchers toyed with the idea of delivering medication through contact lenses. Johnson & Johnson Vision (NYSE:JNJ) Director of Clinica Science Dr. Brian Pall told Drug Delivery Business News that patents back then disclosed how…
J&J expands it long-acting injectables partnership with Midatech
Midatech Pharma (Nasdaq:MTP) announced today that it extended its R&D collaboration with Johnson & Johnson’s (NYSE:JNJ) Janssen Pharmaceuticals. Cardiff, United Kingdom-based Midatech, a drug delivery technology company focused on the biodelivery and biodistribution of medicines, initially entered into the R&D collaboration with Janssen on July 21, 2020. Get the full story at our sister site, Drug Delivery Business News.
Evonik launches new Eudratec tech to boost solubility of oral small molecules
Evonik (Essen, Germany) announced today that it now offers Eudratec SoluFlow, a microparticle technology meant to enhance solubility of active pharmaceutical ingredients in oral drug products. The emulsion-based process technology overcomes solubility hurdles that cannot be resolved by existing manufacturing technologies, according to Evonik. With more than 70% of new small molecules being insoluble, Eudratec…
PharmaJet partner touts interim safety results for needle-free COVID-19 vaccine
PharmaJet announced today that its partner, Technovalia, reported positive interim safety results from a needle-free COVID-19 vaccine trial. Golden, Colorado-based PharmaJet’s needle-free injection systems are being studied with Covigen, a DNA-based vaccine developed by French-Thai pharmaceutical company BioNet-Asia in collaboration with Melbourne, Australia-based Technovalia. Enrollment for the trial began in June 2021. Get the full story at…
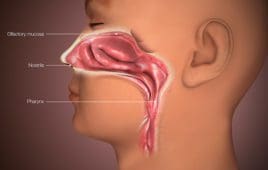
Could nasal vaccines be the next big weapon against COVID-19?
Because of the way they provide protection, nasal vaccines could be the best long-term way to prevent COVID-19 infection, according to experts cited in The New York Times. The Times reported that India-based Bharat Biotech, which has the Covaxin COVID-19 vaccine authorized in India and elsewhere, has an experimental COVID-19 nasal vaccine that may offer…
Gilead Sciences, Merck near the top of Newsweek’s most responsible companies list
A handful of big names in drug discovery and development are among the 500 “most responsible,” according to Newsweek. The outlet published its “America’s Most Responsible Companies 2022” list, marking the third installment of the compilation (in partnership with Statista), this time expanded to include 500 of the largest public corporations around. Companies were judged with an overall…
7 diabetes treatment innovations to look out for on World Diabetes Day
World Diabetes Day — Nov. 14 — centers around raising awareness for those with diabetes. This year, that aim remains the same, and medical technology companies continue to look for ways to continue improving the management of the metabolic disease. Some of those treatments involve insulin, which was discovered as a treatment for diabetes in…
AbbVie, University of Chicago lengthen oncology research partnership until 2025
AbbVie (NYSE: ABBV) has extended an agreement with the University of Chicago related to collaborative preclinical oncology research. The North Chicago, Illinois–based company and the university are working together on research involving biomarkers and therapeutic applications related to existing AbbVie programs. In the past, the organizations have also explored new drug delivery methods to improve…
European regulators offer positive opinion on Janssen’s schizophrenia treatment
Johnson & Johnson’s Janssen Pharmaceutical Companies today announced a positive CHMP opinion on its Byannli schizophrenia treatment. The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) issued the positive opinion. The committee recommended using long-acting atypical antipsychotic Byannli (6-monthly paliperidone palmitate; PP6M) therapy for the maintenance treatment of schizophrenia. The recommendation…
Three companies to collaborate on potential inhaled COVID-19 treatment
Signal Rx Pharmaceuticals, Crystec and ADYA are working on a dry powder drug formulation for potentially treating COVID-19-related illness. The three companies’ collaboration for the dry powder formulation of SF2523 covers the treatment of pulmonary fibrosis, lung cancer and illnesses related to SARS-CoV-2, the virus causing COVID-19. Get the full story at our sister site,…
Purdue researchers tout tuberculosis vaccine development strategy
Researchers at Purdue University and Houston Methodist Research Institute are touting a novel strategy for developing a tuberculosis (TB) vaccine. Bacillus Calmette-Guérin (BCG) is widely used as a vaccine against TB, but the researchers say it has a variable protection against neonatal and adult pulmonary TB, with protection ranging from 0% to 80% among infants.…
Inovio shares down on missed Q2 projections as it moves forward with COVID-19 vaccine
Inovio Pharmaceuticals (NSDQ:INO) shares took a hit this morning on second-quarter results that missed the consensus forecast. INO shares were down more than –11% at $8.53 per share in morning trading today. Get the full story at our sister site, Drug Delivery Business News.
PerkinElmer announces Street-beating Q2, plans to acquire BioLegend for $5.3B
PerkinElmer (NYSE:PKI) today posted Street-beating second-quarter revenues and announced the $5.3 billion acquisition of BioLegend. The Waltham, Mass.-based company posted profits of $245.9 million, or $2.19 per share, on sales of $1.2 billion for the three months ended July 4, 2021, for a 79.3% bottom-line gain on sales growth of 51.3%. Get the full story at…
Annovera offers long-lasting birth control
In 2018, FDA indicated Annovera from TherapeuticsMD to prevent pregnancy. A drug-device combination, Annovera combines a ring-shaped vaginal delivery system with hormones. The manufacturer designed the device to remain in place for three weeks followed by removal for one week. The product is effective for up to one year (or 13 menstrual cycles). The manufacturer…
Eli Lilly acquires glucose-sensing insulin developer Protomer Technologies
Eli Lilly (NYSE:LLY) announced today that it acquired peptide- and protein-engineering platform developer Protomer Technologies. The potential value of the transaction is over $1 billion, depending on the achievement of future development and commercial milestones. Pasadena, Calif.–based Protomer Technologies develops next-generation protein therapeutics designed to identify and synthesize molecules that can sense glucose or other endogenous…
Thermo Fisher Scientific expands corporate leadership team
Thermo Fisher Scientific (NYSE:TMO) announced today that it appointed Alan Sachs to chief medical officer and Karen E. Nelson to chief scientific officer. Sachs served as Waltham, Mass.-based Thermo Fisher’s CSO since 2016 and will be succeeded by Nelson as he assumes the newly established CMO position at the company. Both will join the company’s leadership…
Charles River Laboratories completes $292.5M Vigene acquisition
Charles River Laboratories (NYSE:CRL) announced that it completed a $292.5 million acquisition of Vigene Biosciences. Rockville, Md.-based Vigene, a gene therapy contract development and manufacturing organization (CDMO), develops viral vector-based gene delivery systems, with a primary area of expertise in CGMP viral vector manufacturing for gene therapies and gene-modified cell therapies. Get the full story…