
[Ricky/Adobe Stock]
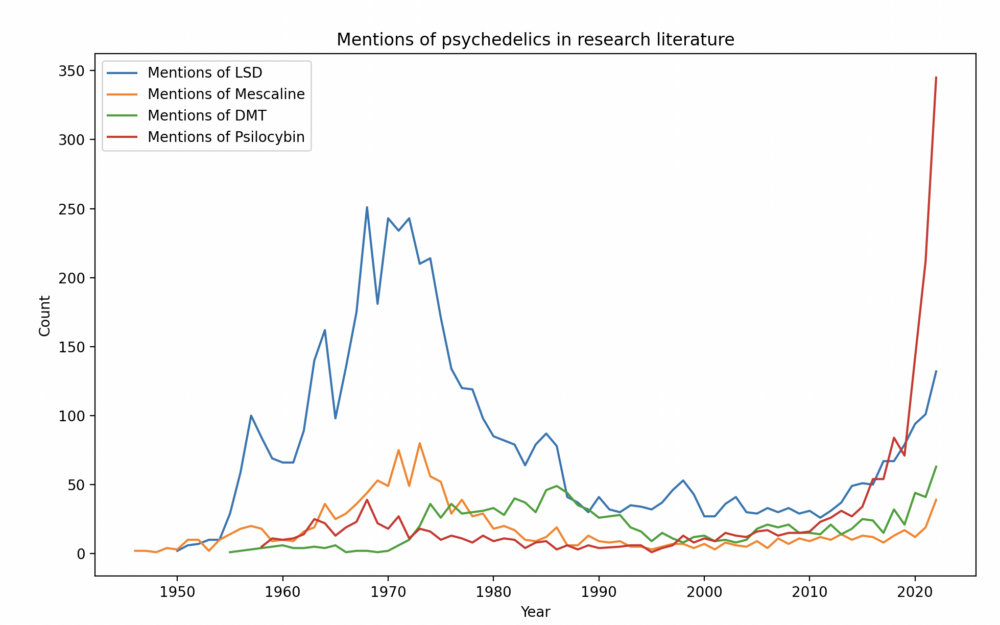
This complex history, along with social and political responses to the countercultural revolution of the 1960s, contributed to a backlash against these substances. Classic psychedelics such as LSD, psilocybin, mescaline, and DMT experienced a swift fall from mainstream favor after the 1960s. Consequently, authorities classified them as Schedule I substances, the most restrictive category, halting research into their effects and medical potential for decades. In recent years, however, regulators have permitted a growing body of research into these drugs.
[Data from PubChem]
Psychedelic drug development considerations
While the compounds offer promise, they remain experimental and unproven, explained Dr. Tiffany Farchione, director of the Division of Psychiatry in the FDA’s Center for Drug Evaluation and Research in a press release. “The goal [of the draft guidance] is to help researchers design studies that will yield interpretable results that will be capable of supporting future drug applications,” Farchione said.
Results from some small studies have been strikingly positive. A study from Johns Hopkins researchers, for instance, found that psilocybin, the active compound in magic mushrooms, significantly cut symptoms of major depression in 24 participants. Half achieved full remission for at least 4 weeks. In a separate study published in NEJM, psilocybin was slightly more effective than the selective serotonin-reuptake inhibitor escitalopram (Lexapro). Compass Pathways plans to conduct two late-stage clinical trials to study psilocybin-assisted therapy for treatment-resistant depression (TRD). It completed the design of one of those trials in late 2022. The company hopes to work with the FDA to approve psilocybin for TRD treatment, possibly by 2025.
The FDA’s guidance on psychedelic drug development covers considerations across several domains:
Chemistry, manufacturing and controls (CMC)
Developers must submit data on the drug’s identity, quality, purity, and strength, including chemistry, manufacturing, and controls (CMC) data. While FDA noted that sponsors can consider drugs from plants or fungi to be botanicals, they must follow Current Good Manufacturing Practice (CGMP).
Nonclinical safety studies
Studies should align with the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) M3(R2) guidance. Psychedelics with prior human exposure may require less initial toxicology; new psychedelics need comprehensive testing first. Binding to 5-HT receptors, especially 5-HT2B, should be evaluated. Studies should support chronic or repeat dosing if needed.
Clinical pharmacology
The agency notes that sponsors must characterize pharmacokinetics, pharmacodynamics, food effects, drug interactions, dose-response and long-term safety must be characterized. It warns that patients with valvulopathy should be excluded as long-term exposure to 5-HT2B agonists may induce this. One study found possible a potential association between 3,4-methylenedioxymethamphetamine (MDMA) abuse and valvular heart disease.
Abuse potential assessment
Sponsors must evaluate Abuse potential with the agency’sAbuse Potential of Drugs guidance. Schedule I psychedelics require DEA registration for studies. Assess dependence potential and collect abuse-related adverse event data.
Clinical Studies
Studies must provide substantial evidence. Placebo controls may be difficult; alternative controls should be considered. Psychotherapy’s role must be clarified. Rigorous safety monitoring, patient risk information, cardiovascular risk evaluation, dose-response assessment, response durability, and repeat dosing safety/efficacy are required.
Risk mitigation and public health effects
Risk mitigation strategies should consider nonmedical use, accidental exposure, and public health risks. A Risk Evaluation and Mitigation Strategy (REMS) may be needed. “While these guidelines are a draft, their publication is a positive signal from the FDA of its commitment to a clear pathway for psychedelic drug development, removing regulatory uncertainty for investors and clinical trial sponsors,” said Doug Drysdale, CEO of Cybin, which is developing a deuterated psilocybin analog among other psychedelics.
“None of these guidelines came as a surprise,” Drysdale added. “Our preclinical and clinical programs have been fully compliant from the start, reinforcing our leadership in making psychedelic compounds available for the treatment of multiple therapeutic indications.”
Following the FDA’s release of draft guidelines aimed at facilitating the development of psychedelic drugs, Drysdale affirmed that the company’s mission and focus remain unwavering. “Our focus remains on improving these compounds — minimizing variability, reducing side effects, shortening treatment sessions, increasing the duration of therapeutic response, and making dosing easier — for the benefit of patients and healthcare providers alike,” he concluded.
Filed Under: clinical trials, Drug Discovery, Psychiatric/psychotropic drugs, Regulatory affairs



