[Updated April 4, 2024] In the face of rising R&D costs and growing pricing pressures from payers, the pharma and biotech sectors continue to transform to adapt to an evolving landscape. While workforce reductions persist in 2024 for some companies, major players like AbbVie, AGC Biologics, and Amgen are demonstrating confidence in the industry with…
Clinical trials have a diversity Problem. Real-world data could be part of the solution
While diversity in clinical trials has won attention in recent years, uneven representation of diverse populations in clinical trials remains a core barrier to global healthcare equity. Real-world data (RWD) paired with artificial intelligence techniques can almost instantly analyze how well drugs work in diverse subpopulations once they hit the market. RWD can also help…
Unlearn CEO: Digital twins could slash clinical trial patient enrollment by 25% or more
The startup Unlearn embodies several trendy AI characteristics. Generative AI company? Check. San Francisco headquarters? Check. Aims to disrupt drug development (specifically, clinical trials) with AI? Check. Prominent AI leadership? Check. Mira Murati, the high-profile CTO of OpenAI, joined Unlearn’s board of directors in 2023. But the company’s pedigree is unique. The firm was founded…
Could Wegovy’s cardiovascular label expansion be a catalyst for GLP-1 obesity drug coverage?
The recent FDA approval of a cardiovascular risk reduction indication for Wegovy (semaglutide) could point toward a significant opportunity for pharma companies seeking to reshape payer perceptions and expand coverage for next-gen metabolic therapies. This regulatory shift, allowing Wegovy to be prescribed for reducing the risk of major adverse cardiovascular events such as heart attack…
FDA fast-tracks psilocybin-based CYB003 for depression
The FDA continues to signal openness to psychedelic-based therapies. Following its recent Breakthrough Therapy Designation (BTD) for a potential LSD-based anxiety treatment, the agency has extended the same status to Cybin Inc.‘s CYB003 for major depressive disorder (MDD). The move marks the first FDA Breakthrough Therapy Designation for an adjunctive, psychedelic-based Major Depressive Disorder treatment.…
Moving the needle on diversity in clinical trials: Where do we go from here?
Enhancing patient diversity in clinical trials has become a key priority in drug development. The main concern is that critical data that includes underrepresented patient populations is being left out as many clinical trials do not reflect all populations that may eventually take a therapy. These underrepresented groups consist of women, including those who are…
Amidst empty labs, signs of biotech’s resurgence emerge
In 2023, a year of accelerated regulatory success, a significant number of biotech labs sat empty in major hubs like San Francisco and Boston. The FDA approved 55 novel therapies in 2023, including Leqembi for early Alzheimer’s and Zurzuvae for postpartum depression. The approval number marked the second highest count in three decades (see graph…
Unifying disparate data in clinical trial management with advanced data technology
The increasing complexities in clinical trials, including expanded patient populations, decentralized trials and new technologies, are directly impacting the amount of data and information available to clinical trial sponsors. Though this provides significant value to the industry, it presents a new challenge of consolidating disparate and siloed systems, improving standardization and unifying the explosion of…
Core trends in 2023 FDA drug approvals: Oncology, neurology and hematology dominate
2023 was a big year for hematology, neurology and oncology, with the medical specialties seeing the most FDA approvals. In terms of sponsors, Pfizer had the most approvals with six total, followed by UCB and Chiesi, each with three apiece. When looking at commercial prospects, AstraZeneca’s respiratory syncytial virus antibody Beyfortus could be the biggest…
2024 forecast: Navigating new frontiers in pharma with AI, synthetic data, and strategic partnerships
In late 2022, we published a series of predictions, which, among other things, projected that 2023 would be a “massive showcase” for machine learning (ML) in drug discovery and development. And in many ways, 2023 was a pivotal year for AI in pharma. This evolution in AI and ML applications in pharma built upon the…
Eversana’s AWS alliance aims to cut red tape in pharma regulatory paperwork
In July, the life sciences services company Eversana revealed it was partnering with AWS to tap generative AI (gen AI) for pharma and other life science customers. It aimed to “pharmatize” gen AI, as the company put it then. Now, the company has revealed the first technology from that partnership – a regulatory review automation…
Vaccine mega-trials: Rare behemoths in the vaccine trial landscape
Abstract The vast majority of vaccines are prophylactic in nature. As a result, the demonstration of their efficacy paradoxically requires the infectious disease to occur among non-diseased study participants randomized between investigational vaccine and appropriate control groups. The statistics of vaccine efficacy (VE) calculation are nearly entirely and solely based on the number of observed…
Anticipating IND submission: Ensuring your drug is ready for preclinical toxicology studies
Drug development is often a vast and intricate journey. Each phase signifies an advancement in the process, always with an eye toward patient safety and efficacy. But before any therapeutic finds itself on the bedside tables of hopeful patients, it faces a formidable challenge: preclinical toxicology testing. As the gateway to clinical trials, this early…
FTC cracks down on 100-plus ‘improper’ drug patents in comprehensive enforcement push
Under the leadership of Lina Khan, the Federal Trade Commission (FTC) has stepped up its enforcement actions, complicating its clearances of mergers, including Amgen’s acquisition of Horizon Therapeutics, which concluded in early October. Most recently, FTC has challenged what it deems are “improper” patent listings. At the receiving end of the FTC drug patent crackdown…
NLP in drug discovery and the quest for the ‘right’ research elements
In drug discovery and development, data sources are as diverse as they are plentiful. There are comprehensive databases brimming with molecular targets, cellular processes, genomic sequences, proteomic profiles, and metabolite patterns that shed light on disease pathways. Data possibilities in the patient care realm are similarly vast, spanning electronic medical records, imaging datasets, and even…
Novo Nordisk stops once-weekly semaglutide kidney outcomes trial early following interim analysis
Novo Nordisk will halt the phase 3b FLOW trial, which investigated the effects of once-weekly injectable semaglutide on kidney outcomes in individuals with type 2 diabetes (T2D) and chronic kidney disease (CKD). The company reached the decision following the recommendation of the independent Data Monitoring Committee (DMC), following an interim analysis that met pre-specified criteria…
Upadacitinib shows promising results in ongoing phase 3 atopic dermatitis studies
AbbVie has shared new efficacy and safety data for Rinvoq (upadacitinib) in adults and adolescents with atopic dermatitis (AD) from a trio of ongoing phase 3 studies. Spanning 140 weeks, these studies sustained the co-primary endpoints of Eczema Area and Severity Index 75 (EASI 75) and validated Investigator’s Global Assessment for Atopic Dermatitis 0/1 (vIGA-AD…
Do rising interest rates actually fuel pharma M&A? Sometimes, yes
Conventional wisdom holds that rising interest rates curb M&A activity, but an analysis of the pharma and biotech M&A data from 2018 to early October 2023 reveals a counterintuitive trend — at least over the short term. As interest rates have risen in 2023, pharma deal-making has remained strong although the deal size has dipped.…
More than a century after its synthesis, MDMA could be headed for FDA approval for PTSD
First synthesized in 1912 by Merck, the empathogenic drug 3,4-Methylenedioxymethamphetamine (MDMA) is inching toward FDA approval following the positive results of a phase 3 study. The recently concluded phase 3 study, MAPP2, published in Nature Medicine, found that MDMA-assisted therapy significantly outperforms traditional talk therapy in reducing PTSD symptoms. Participants receiving MDMA-AT had an 86.5%…
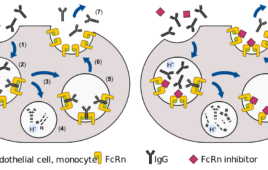
The multi-billion dollar promise of efgartigimod and the broader FcRn inhibitor market
Thanks to promising candidates such as efgartigimod, the Fc receptor (FcRn) inhibitor market is poised to reshape the autoimmune disease treatment landscape. Sales projections for the market top $10 billion, as Driehaus Capital Management estimated in 2020. The addressable U.S. patient base spans more than 228,500 individuals across various conditions including myasthenia gravis, warm autoimmune…
Biotech sees new shoots of growth but has thorns left to prune
Today, Boston-based Ginkgo Bioworks’ recently unveiled a deal with Pfizer on RNA-based drug candidates, reflecting the growing interest in RNA therapies. Additionally, Carlsbad, California–based Ionis Pharmaceuticals inked a pact with Roche focused on RNA-targeted therapies for Alzheimer’s and Huntington’s diseases. This development underscores the resilience of prominent biotech hubs. According to data from real estate…
High-profile drugs under the legal spotlight in 2023
In the wake of the COVID-19 pandemic, the U.S. has witnessed a surge in litigation. One factor driving the uptick in the pharmaceutical sector is the rise of copycat litigation. As mass tort activity amplifies, plaintiff lawyers are treating previously filed lawsuits as templates for new claims. Examples of the trend include steadily increasing nitrosamines…
Sanofi ALTUVIIIO hemophilia A treatment: FDA accepts sBLA based on pediatric phase 3 data
The FDA has accepted Sanofi’s supplemental Biologics License Application (sBLA) for ALTUVIIIO, a novel, high-sustained factor VIII replacement therapy for adults and children with hemophilia A. The FDA based the decision on positive final data from the pivotal Phase 3 XTEND-Kids trial in children under 12 with hemophilia A. ALTUVIIIO (efanesoctocog alfa) is the first…
Pfizer and Moderna win FDA nod for XBB.1.5 COVID-19 vaccine boosters, but projected sales pale in comparison to 2022’s
As Pfizer and Moderna receive the FDA nod for COVID-19 boosters, their 2023 global sales projections appear to be just a fraction of the previous year’s. In 2022, Pfizer and its partner BioNTech jointly sold $56 billion worth of their Comirnaty COVID-19 vaccine, marking the best-selling drug of the year. Moderna’s Spikevax COVID-19 vaccine wasn’t…
Top 12 reported events for Moderna and Pfizer omicron-targeting COVID-19 boosters
With updated COVID-19 boosters set to launch in the near future and COVID-related hospitalizations on the rise again, scrutiny is on the rise of emerging variants and the benefit-safety profile of COVID-19 vaccines. We performed a retrospective analysis of data from the Vaccine Adverse Event Reporting System (VAERS), focusing on the period covering the launch…