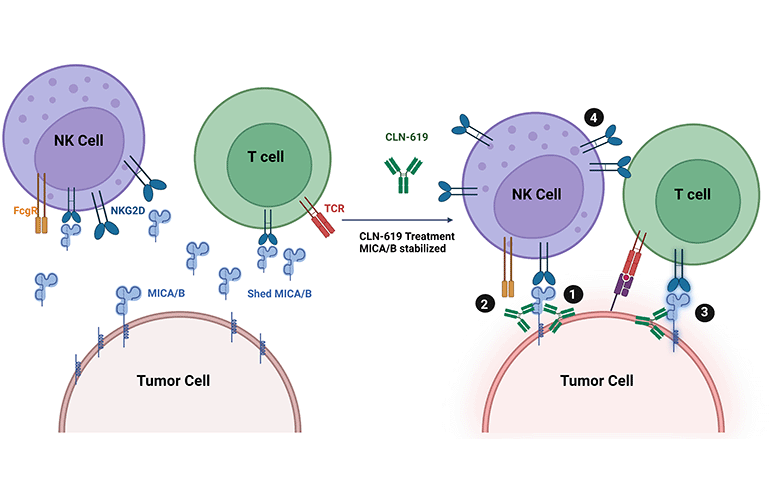
Illustration of Fragment Crystallizable (Fc)-mediated cytotoxicity boosting tumor cell destruction. It highlights the increased MICA-NKG2D interaction, enhancing NK cell/T cell activation, and shows the reduction of MICA/MICB decoy release, improving NKG2D functionality. [Image courtesy of Cullinan Oncology]
Dr. Judy Wang, Associate Director of Drug Development at the Florida Cancer Specialists and Research Institute, explained the science behind the therapy. “CLN-619 is a human IgG1 monoclonal antibody that binds to cell surface ligands called MICA and MICB. These new immune therapies are trying to overcome the tactics that tumors use to hide from detection by the immune system,” she said.
Current limitations of traditional treatments for gynecological malignancies
Traditional treatments for patients with advanced gynecological malignancies, such as platinum chemotherapy agents, often yield modest clinical benefits but are accompanied by high toxicity. This reality underscores the critical need for more effective treatments that maintain a safer, tolerable profile and can be administered over extended periods.
In this context, Wang expressed her optimism about the performance of CLN-619 in the phase 1 study, especially with gynecological endometrial patients. “We’re excited about some of the emerging clinical data that we’re seeing in our gynecological endometrial patients who are achieving at least a partial response at the time of data cutoff,” Wang said. “It seems that this mechanism might be a good target potentially for clinical benefit and that there may be something to the biology of these tumors, particularly these gynecological cancers, that would better enhance natural killer and T cell engagement versus other aspects of the immune system or even with other tumor types.”
The clinical trial modules for CLN-619: Single and combined therapies
The trial is organized into two modules. Module A involves dose escalation and cohort expansion of CLN-619 alone, whereas Module B examines dose escalation and cohort expansion of CLN-619 in combination with pembrolizumab.
Wang indicates that CLN-619 was well-tolerated overall, with primarily grade two or lower infusion-related adverse events such as fever, abdominal pain, nausea and fatigue, all of which were manageable. There were no grade three or four treatment-related adverse events reported.
| Category | All Patients (n=37) | Response Evaluable¹ at ≥1 mg/kg (n=22) | Response Evaluable¹ GYN Malignancy² (n=10) |
|---|---|---|---|
| Complete Response (CR) | 1 | 1 | 0 |
| Partial Response (PR)³ | 2 | 2 | 2 |
| Stable Disease (SD) | 7 | 7 | 5 |
| CR + PR + SD | 10 | 10 | 7 |
| Progressive Disease (PD) | 18 | 12 | 3 |
| Not Evaluable (NE) | 9 | NA | NA |
| ¹Patients who underwent at least one RECIST response assessment or who had clinically assessed PD prior to first planned response assessment. ²Endometrial, cervical and ovarian. ³The observed partial responses were unconfirmed but ongoing at the time of data cut-off. |
|||
Wang also spoke of encouraging results from the Phase 1 data. “We are starting to see some emerging clinical activity. There’s definitely an unmet need for efficacious therapy for these advanced GYN patients, particularly those diagnosed late,” Wang added.
“What’s unique about 619 is its potent engagement at the level of NK and CD cells through antibody-derived cytotoxicity. We may be discovering a means that the immune system hasn’t found in previous immunotherapies to further reengage and reactivate immune activation and cell killing,” stated Wang.
Current progress of CLN-619 trials and the role of tumor biopsies

Dr. Judy Wang
Wang also gave an update on the current trial progress, stating, “We’ve completed the initial dose escalation phase, and we are actively enrolling two expansion cohorts in both cervical and endometrial for the monotherapy. Additionally, we’re also looking at combinations of CLN-619 with pembrolizumab, which is actively accruing as well.”
Notably, the research team is also using tumor biopsies to further understand the effects of the therapy. “We’re actively collecting paired tumor biopsies for pharmacodynamic and biomarker assessments, and once we’ve been able to analyze that information, it may help guide us into what will be good combinations based on a mechanistic pattern of what we’re seeing in the tissue,” shared Wang.
The potential of CLN-619 is a testament to the power of immunotherapy. “It’s evidence of the potential of re-engaging the immune system and finding other avenues outside of conventional checkpoint inhibition to induce cytotoxic cell killing,” Wang explained.
Expressing her enthusiasm for the trial, Wang concluded, “This drug, in particular, is exciting; it’s a unique target and we’re seeing clinical activity amidst a safe and tolerable profile. It kind of ticks all the right boxes which is really encouraging.”
Exploring the potential of CLN-619 and pembrolizumab combination therapy
Reflecting on the promising potential of immunotherapy combinations, Wang highlighted the natural pairing of immuno-oncology agents and checkpoint inhibitors like pembrolizumab. “This combination has been successful in treating several types of tumors as a monotherapy. Engaging the immune system through not just serial, but parallel pathways, might offer a synergistic effect in terms of immune activation,” she remarked.
This combination therapy of CLN-619 and pembrolizumab might further enhance or rekindle a prior checkpoint inhibitor response that has since become ineffective, Wang said. “We’re considering whether we can achieve this, even in certain tumor types that we wouldn’t expect to respond to single-agent immunotherapy. The potential for combinatorial effects of Pembrolizumab and CLN-619 is a question worth answering,” she said.
Regarding the ideal cancer types for this strategy, that remains a subject of continued investigation. “We’re currently collecting paired tumor biopsies for pharmacodynamic and biomarker assessments,” Wang said. Once her team has gathered and analyzed this data, it could help guide them toward the most effective combinations, based on the mechanistic patterns they observe in the tissue. “This information could also help us identify which disease groups might respond better than others to these therapies,” she said. “The journey to discover these answers is ongoing, but the possibilities for patient treatment are promising.”
Filed Under: clinical trials, Drug Discovery, Drug Discovery and Development, Immunology, Oncology



