Sanofi revealed that its investigational BTK inhibitor rilzabrutinib notched a significant win in the LUNA 3 phase 3 study, hitting the primary endpoint of durable platelet response in adults with persistent or chronic immune thrombocytopenia (ITP). The study showed a significantly higher proportion of rilzabrutinib-treated patients achieved the platelet response goal compared to placebo in…
Best-selling pharmaceuticals of 2023 reveal a shift in pharma landscape
Note: This feature on the best-selling pharmaceuticals of 2023 was updated on March 27. Changes are possible as more data become available. Sales of drugs with more than one developer are added together (as in the case of the COVID-19 vaccine Comirnaty) unless one of those companies records the net sales for it, i.e., Dupixent.…
Culmination Bio partners with Merck on disease-agnostic patient data
The bioinformatics startup Culmination Bio had received financial backing from Merck’s venture arm. Now, the Utah-based company is collaborating directly with the pharmaceutical giant on an autoimmune disease research project. According to Culmination Bio‘s CEO Dr. Lincoln Nadauld, the collaboration highlights the value of the startup’s ability to provide rapid access to high-quality longitudinal patient…
Lumen Bioscience cracks the code on spirulina as a biologics factory for c. diff, metabolic disease and more
Clostridioides difficile, commonly known as C. diff, is a significant health threat in the U.S. Recent estimates suggest that C. diff, a common bacteria, can cause infection in roughly 500,000 patients annually in the U.S., with around 30,000 of these cases resulting in death. “Actually, it’s more like 5 million when you think about it…
What’s next for biotech? Q4 2023 funding trends point beyond the usual suspects
Oncology may continue be one of the hottest sectors across the pharma sector, but other therapeutic areas are catching up in terms of innovation and investment. While oncology and hematology jointly accounted for about one-third of the new FDA approvals in 2023, investors are increasingly betting on precision medicine, advanced drug delivery systems and the use of…
Zai Lab’s unified approach to tackling cancer, autoimmune, and neurological diseases
Zai Lab is a global biopharmaceutical company founded in 2014 and based in China and the U.S.. Led by Chairperson and CEO Dr. Samantha Du, the company now has more than 2,000 employees internationally. Focusing on developing novel therapies for oncology, autoimmune disorders, infectious diseases, and neurological disorders, it has built a broad pipeline of…
Using AI to unlock new uses for existing cancer medicines
Repurposing is a drug development strategy that has been widely applied in cancer. This strategy, sometimes called label expansion, involves obtaining FDA approval to market a drug for the treatment of new indications, alone or in combination with other drugs. Not only can this approach extend the window of patent protection for a commercialized drug,…
How a J&J exec found her calling in autoantibody drug development
Dr. Katie Abouzahr’s career, which began in the wards of the UK’s National Health Service (NHS) before extending into management consulting, paved the way for her leadership of autoantibody and maternal fetal medicine programs at Johnson & Johnson. “It’s not a typical pharma executive’s straight line path,” she acknowledged. “Careers can often be jungle gyms…
Nipocalimab shows promise in RA subgroups in phase 2a IRIS-RA study
Johnson & Johnson’s nipocalimab, which works by targeting the neonatal Fc receptor (FcRn), has the potential to treat an array of autoimmune conditions. But the antibody recently hit a snag in the first-ever clinical study of an FcRn inhibitor in rheumatoid arthritis (RA), missing its primary endpoint. The development has sparked debate within the rheumatology…
Vaccine mega-trials: Rare behemoths in the vaccine trial landscape
Abstract The vast majority of vaccines are prophylactic in nature. As a result, the demonstration of their efficacy paradoxically requires the infectious disease to occur among non-diseased study participants randomized between investigational vaccine and appropriate control groups. The statistics of vaccine efficacy (VE) calculation are nearly entirely and solely based on the number of observed…
Unlocking the secrets of cellular immunogenicity: A deep dive in ELISpot assays
Next generation therapies and vaccine research have recently dominated headlines and scientific discovery. This research and its ability to treat previously untreatable conditions has rightfully excited the scientific and medical communities worldwide. But the complexities involved require a nuanced approach to ensure the treatment is safe and effective. Integrating cell culture into cellular immunity testing…
Click chemistry breakthroughs drive Shasqi and J&J cancer alliance
In June 2023, the click chemistry-focused startup Shasqi revealed a research pact with Johnson & Johnson Enterprise Innovation. More recently, the company announced that it had expanded the research alliance, furthering work on its intratumorally injected biopolymer, known as SQL70. The collaboration will also apply its clinically validated Click Activated Protodrugs Against Cancer (CAPAC) technology…
Upadacitinib shows promising results in ongoing phase 3 atopic dermatitis studies
AbbVie has shared new efficacy and safety data for Rinvoq (upadacitinib) in adults and adolescents with atopic dermatitis (AD) from a trio of ongoing phase 3 studies. Spanning 140 weeks, these studies sustained the co-primary endpoints of Eczema Area and Severity Index 75 (EASI 75) and validated Investigator’s Global Assessment for Atopic Dermatitis 0/1 (vIGA-AD…
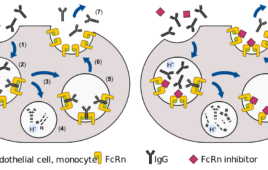
The multi-billion dollar promise of efgartigimod and the broader FcRn inhibitor market
Thanks to promising candidates such as efgartigimod, the Fc receptor (FcRn) inhibitor market is poised to reshape the autoimmune disease treatment landscape. Sales projections for the market top $10 billion, as Driehaus Capital Management estimated in 2020. The addressable U.S. patient base spans more than 228,500 individuals across various conditions including myasthenia gravis, warm autoimmune…
Johnson & Johnson pharma rebrand highlights innovation as a pillar to reinforce trust
Global pharma and medical device giant Johnson & Johnson (J&J) has ditched its iconic cursive logo that dates back to the late 19th century, and rebranded its Janssen pharma division as Johnson & Johnson Innovative Medicine. The move underscores the company’s push to prioritize higher-margin prescription drugs. This strategic move comes amidst a backdrop of…
Top 12 reported events for Moderna and Pfizer omicron-targeting COVID-19 boosters
With updated COVID-19 boosters set to launch in the near future and COVID-related hospitalizations on the rise again, scrutiny is on the rise of emerging variants and the benefit-safety profile of COVID-19 vaccines. We performed a retrospective analysis of data from the Vaccine Adverse Event Reporting System (VAERS), focusing on the period covering the launch…
How COVID vaccine options stack up for fall 2023
[Updated September 7. Article originally posted on July 21, 2023. Updates follow in bold:] The Vaccines and Related Biological Products Advisory Committee (VRBPAC) is backing a significant shift in the current COVID-19 vaccine strategy: a move from multivalent to monovalent vaccines focusing on the XBB lineage strains. More recently, the emergence of new variants such…
Moderna and Pfizer ready updated COVID-19 boosters to combat BA.2.86 and other emerging variants
Against the backdrop of a nearly 16% spike in COVID-19 hospitalizations in late August, according to CDC data, federal authorities are gearing up to greenlight updated boosters. Moderna announced that its latest COVID vaccine is effective against this new strain. Meanwhile, Pfizer revealed positive preclinical data for its vaccine, developed in collaboration with BioNTech. In…
Five insights on COVID-19 vaccine side effects
To date, there have been more than 770 million confirmed COVID-19 cases and nearly 7 million deaths from the virus, according to the World Health Organization (WHO). Vaccines remain one of the most potent tools in blunting the severity of SARS-CoV-2 infection, and vaccine developers have distributed more than 13.5 billion vaccine doses to date.…
Epstein-Barr virus: Trigger and driver of multiple sclerosis?
Recent research has indicated a link between Epstein-Barr virus (EBV) and multiple sclerosis (MS), with some researchers going as far as to say that EBV might be a potential trigger of MS. However, it remains unclear whether the virus also drives the progression of the disease. Current treatments focus largely on moderating inflammation. In this…
‘Long vax’ phenomenon gets closer look in recent studies
In 2020, researchers witnessed the emergence of post-acute sequelae of SARS-CoV-2 (PASC) — more commonly referred to as “long COVID.” Now, the notion of “long vax,” persistent and varying symptoms following COVID-19 vaccination, has come into focus, as Science has noted. This phenomenon, while not as widespread as long COVID, has concerned some in the…
Guselkumab could offer hope for psoriatic arthritis patients resistant to TNF inhibitors
Janssen continues to strengthen the case that its interleukin-23 inhibitor TREMFYA (guselkumab) offers promise to many patients with psoriatic arthritis (PsA). The company’s phase 3b COSMOS clinical trial involving 189 patients with active PsA and an inadequate response to one to two previous tumor necrosis factor (TNF) inhibitors, guselkumab showed sustained improvements in measures of…
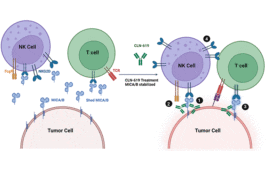
CLN-619 antibody therapy offers a new hope for patients with advanced solid tumors
Boston-based Cullinan Oncology has unveiled data for its new monoclonal antibody therapy, CLN-619, ahead of the American Society of Clinical Oncology (ASCO) 2023 meeting scheduled for June 2–6 in Chicago. The drug could potentially offer a new treatment option for patients with advanced solid tumors. Dr. Judy Wang, Associate Director of Drug Development at the…
The growing influence of digital pathology in preclinical R&D: Spotlight on Proscia’s Concentriq
As drug discovery evolves, digital pathology platforms are playing a vital role in streamlining preclinical R&D processes and accelerating drug discovery. Proscia‘s Concentriq for Research reflects this trend. Earlier this year, we profiled PathAI’s AISight, a digital pathology platform designed to support AI-driven research. To learn more about Proscia’s digital pathology technology, we caught up with…
Unraveling the impact of FDORA and PREVENT Pandemics Acts on the life sciences
As the world continues to grapple with global health challenges, the role of science and biotech law has taken center stage in shaping public health policy and innovation. The FDORA and PREVENT Pandemics Acts are poised to help shape the landscape. In a recent interview, life sciences attorney Barbara Binzak Blumenfeld offers insights into significant…