The FDA has signed off on two novel therapies targeting rare pediatric cancers. Novartis’ Lutathera targets aggressive gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in children 12 and up, while Day One Biopharma’s Ojemda (tovorafenib) tackles treatment-resistant BRAF-mutated relapsed or refractory pediatric low-grade glioma (pLGG) with a BRAF fusion or rearrangement, or BRAF V600 mutation These approvals offer…
Rilzabrutinib on track for regulatory filing after ITP trial win
Sanofi revealed that its investigational BTK inhibitor rilzabrutinib notched a significant win in the LUNA 3 phase 3 study, hitting the primary endpoint of durable platelet response in adults with persistent or chronic immune thrombocytopenia (ITP). The study showed a significantly higher proportion of rilzabrutinib-treated patients achieved the platelet response goal compared to placebo in…
How the FDA approval of J&J’s Opsynvi could simplify treatment and improve outcomes for PAH patients
Pulmonary arterial hypertension (PAH), a rare, progressive and life-threatening blood vessel disorder, affects some 500 to 1,000 new patients each year in the U.S. FDA recently approved Opsynvi, a first-of-its-kind once-daily single-tablet combination therapy from Johnson & Johnson. “With this approval, our portfolio now includes treatments that address all three guideline-recommended pathways,” said a J&J…
Global biotech VC trends in Q1 2024
The first quarter of 2024 saw a flurry of funding activity in the biotech sector, with early-to-mid-stage companies attracting most of the investment dollars. Among the most notable late-stage deals was blood-based cancer diagnostics firm Freenome‘s $254 million Series E round. The South San Francisco-based company, which is developing a platform for early cancer detection…
Unleashing a new frontier: The power of germline clinico-genomic data to drive therapeutic development
Over the past decade, the use of deeper sources of real-world data across all stages of the drug development life cycle has become increasingly important to guide disease understanding, trial designs, clinical guidelines, regulatory submissions and post-market studies. The advent of these deeper sources was prompted by the HITECH Act, which had the effect of…
Genentech’s lab in the loop aims to tap the power of quantity for quality drug discovery
We can design chips that power self-driving cars and create physically-realistic video footage based on text descriptions. Yet, as Genentech’s Aviv Regev pointed out in a session about the company’s lab in the loop at NVIDIA’s GTC conference, the humble cells within us operate with a complexity that still eludes our full understanding. It turns out that a cell is itself like a computational device…
Q&A: How Insilico Medicine’s AI identified a new IPF drug target in record time
Idiopathic Pulmonary Fibrosis (IPF), a devastating lung disease affecting millions with increasing incidence, may have a new treatment hope thanks to a novel inhibitor of TNIK, a kinase newly implicated in fibrosis, identified using generative AI drug discovery platforms in just 18 months. Researchers at Insilico Medicine, along with international collaborators, harnessed the power of…
BIOiSIM assigns ‘credit scores’ to drug candidates
VeriSIM Life, a San Francisco-based startup, has created BIOiSIM, an AI-powered platform that simulates drug compound behavior in the human body by acting like a virtual laboratory. It assigns each a predictive “credit score” predicting its viability for drug development. “It’s like a FICO score for drug development,” founder and CEO Dr. Jo Varshney said.…
What’s next for biotech? Q4 2023 funding trends point beyond the usual suspects
Oncology may continue be one of the hottest sectors across the pharma sector, but other therapeutic areas are catching up in terms of innovation and investment. While oncology and hematology jointly accounted for about one-third of the new FDA approvals in 2023, investors are increasingly betting on precision medicine, advanced drug delivery systems and the use of…
Insilico’s AI-discovered INS018_055 graduates to phase 2
Roughly a year ago, Insilico Medicine announced that it had dosed the first patient in a phase 1 study of INS018_055, an AI-discovered, first-in-class small molecule inhibitor. Now, the company has progressed to the next stage, launching a phase 2 study for the drug candidate. Insilico, a founding member of NVIDIA Inception, developed its AI…
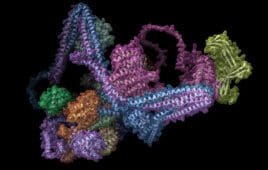
Decoding the enigma of the commander complex: Employing AlphaFold2 to illuminate biological structures
Machine learning algorithms, such as Alphabet’s neural network-based model AlphaFold2, are steadily transforming medical research, shedding light on complex biological structures. A recent case in point involves research using the technology to explore the Commander complex, a 16-protein complex crucial for cellular protein transport processes. This complex is not only vital for normal cellular function,…
HELIOS trial Q&A: Amylyx Pharmaceuticals’ AMX0035 as a potential treatment for Wolfram syndrome
The recently launched phase 2 HELIOS clinical trial from Amylyx Pharmaceuticals, aims to explore the potential of AMX0035 (sodium phenylbutyrate and taurursodiol) in treating Wolfram syndrome, a rare and complex genetic disorder. In a recent interview, Drs. Lahar Mehta and Fumihiko Urano discussed the AMX0035 Wolfram syndrome trial design, objectives and implications for future research…
Chiesi Farmaceutici acquires Amryt Pharma to bolster access to rare disease therapies
Chiesi Farmaceutici, a family-owned international pharma company, has completed its $1.25 billion acquisition of rare disease-focused biopharma Amryt Pharma (Nasdaq:AMYT). The companies announced the deal in January and projected it would close in the first half of 2023. Strengthening Chiesi’s global rare disease focus Chiesi’s decision to acquire Amryt Pharma is part of a broader…
Genomenon collaborates with rare disease foundations on drug development
The AI-driven genomics company Genomenon has announced that it will work with COMBINEDBrain, SynGAP Research Fund and SLC-6A1 Connect. COMBINEDBrain is a 501(c)(3) company focused on developing new treatments for neurodevelopmental disorders. COMBINEDBrain partners with more than 30 neurodevelopmental rare disease foundations, including SynGAP Research Fund and SLC6A1 Connect. “Our collaboration with Genomenon represents a big step toward finding…