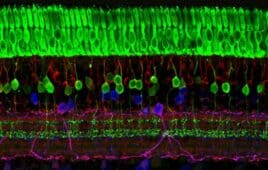
Applied Genetic Technologies Corp. (NSDQ:AGTC) has announced 12-month data from a Phase 1/2 trial involving achromatopsia, an inherited retinal condition affecting roughly 27,000 people in the U.S. and EU. Most people with achromatopsia are legally blind.
Applied Genetic Technologies Corp. (NSDQ:AGTC) has announced 12-month data from a Phase 1/2 trial involving achromatopsia, an inherited retinal condition affecting roughly 27,000 people in the U.S. and EU. Most people with achromatopsia are legally blind.
AGTC is focusing on two gene mutations linked to the disorder known as A3 and B3. The former accounts for roughly half of the cases of achromatopsia while B3 is implicated in about one-quarter.
The company said the ACHM B3 drug candidate shows promising signs of biologic activity based on two visual function measurements. The company acknowledged that there was presently no consistent efficacy signal of activity for ACHM A3, but that it saw encouraging patient anecdotes in the A3 cohort.
Higher dose levels in the trials were correlated with higher response rates.
“Based on the data presented today, an important differences in our construct and clinical trial design, we are confident in our achromatopsia position and have multiple points of differentiation compared to others developing achromatopsia therapies,” said Sue Washer, CEO of AGTC in a investor webinar. The company has conducted more preclinical research and has explored a wider dose range than other organizations developing achromatopsia drugs.
“The results regarding responders in the ACHM B3 trial are encouraging,” said Dr. Rachel Huckfeldt, assistant professor of ophthalmology at Harvard Medical School, in a statement.
The company believes that the A3 version of the drug may offer benefits to pediatric patients. Pediatric research involving both A3 and B3 is underway.
AGTC plans to conduct later-stage trials focusing on the B3 drug candidate.
The company reported no serious adverse events associated with the experimental drug.
Filed Under: clinical trials, Drug Discovery, Ophthalmology