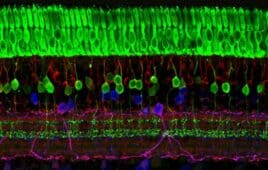
Vision, one of our most valued senses, is a complex orchestration of light-sensing photoreceptors, neurons, and intricate pathways leading to the brain. Central to this native biological system are the photoreceptors – the rods and cones – cells that are responsible for capturing light and initiating visual perception. For many with inherited retinal diseases (IRDs),…
Novel therapy shows promise in improving corneal edema treatment
Cataracts are one of the most common ailments of an aging population, manifesting in the fifth or sixth decade of life. This condition, which leads to blurred or cloudy vision, is caused by proteins that break down in the lens of our eyes, forming an increasingly opaque mass. Although cataracts can cause blindness if untreated,…
Astellas Pharma’s $1.7B Iveric Bio buy aims to bolster its ophthalmology market position
Astellas Pharma, the second largest Japanese pharma firm after Takeda, plans on scooping up the biopharma Iveric Bio for roughly $1.7 billion. Iveric focuses on discovering and developing novel therapies for retinal diseases, for approximately $1.7 billion. Astellas believes the acquisition of Iveric Bio will strengthen its standing in the ophthalmology market, by adding the…
Verana Health launches Qdata anti-VEGF market tracker for in-depth market analysis
Digital health company Verana Health (San Francisco) has unveiled the Qdata anti-VEGF market tracker. It is their first market tracking Data as a Service (DaaS) product. Excessive amounts of vascular endothelial growth factor (VEGF) can cause abnormal blood vessel growth in the eyes, which, when untreated, can result in vision loss in patients with retinal diseases.…
AbbVie and Capsida join forces to target serious eye diseases
AbbVie (NYSE:ABBV) will work with the gene therapy platform company Capsida Biotherapeutics (Thousand Oaks, California) to develop serious eye diseases with few treatment options. The partnership will unite AbbVie’s development and commercialization capabilities with Capsida’s high-throughput adeno-associated virus (AAV) engineering platform. AbbVie will also help explore therapeutic cargo approaches and provide expertise in ophthalmology disease biology.…
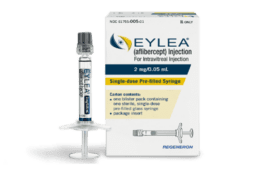
Regeneron’s Eylea approved for preterm infants with ROP, first drug treatment for condition
Regeneron Pharmaceuticals (Nasdaq:REGN) has received an indication for Eylea (aflibercept) injection for preterm infants with retinopathy of prematurity (ROP). The approval is the first pharmacologic treatment for the condition, which affects roughly 50% to 70% of infants with a weight less than 1250 grams (2.76 pounds) at birth. The condition is more common in infants…
FDA grants priority review of aflibercept in retinopathy of prematurity
Regeneron Pharmaceuticals (Nasdaq:REGN) has announced that the FDA has granted Priority Review of the supplemental Biologics License Application (sBLA) for Eylea (aflibercept) Injection to treat retinopathy of prematurity (ROP) in preterm infants. First approved in 2011 to treat wet age-related macular degeneration, aflibercept is a blockbuster drug. Last year, it generated $9.2 billion for Regeneron…
High-dose aflibercept supports sustained vision improvement in DME and wet AMD
Increasing the dose of Regeneron‘s and Bayer‘s aflibercept (Eylea) to 8 mg led to sustained treatment intervals in patients with neovascular eye disease, according to the Phase 2/3 PHOTON and Phase 3 PULSAR clinical trials. In both studies, dosing patients at the 8 mg dose level every 12 or 16 weeks achieved noninferiority compared to 2 mg every 8…
Two-year data show Roche’s Vabysmo improved vision in people with wet age-related macular degeneration
Roche (SIX:RO, ROG; OTCQX:RHHBY) has announced new positive two-year data from its TENAYA and LUCERNE studies of Vabysmo (faricimab) in neovascular or “wet” age-related macular degeneration (nAMD). Based on the study results, Roche estimates that more than 60% of people taking the drug could receive an injection of facrimab once every four months while achieving…
FDA approves generic for dry-eye treatment Restasis
Viatris (NSDQ:VTRS) has received FDA approval for the first generic of Restasis (cyclosporine ophthalmic emulsion) eye drops. FDA first approved the drug, which was developed by Allergan, in 2003. Now marketed by AbbVie (NYSE:ABBV), Restasis sales are on the order of $1.2 billion annually. AbbVie recently noted in its projections for 2022 that it expected…
Novartis to pay up to $1.5 billion to acquire Gyroscope Therapeutics
Novartis (NYSE:NVS) has entered into an agreement to acquire U.K.-based Gyroscope Therapeutics, which specializes in ocular gene therapy company. The acquisition would add GT005, an investigational gene therapy for geographic atrophy, to its pipeline. Geographic atrophy is an eye-sight-threatening condition occurring in some patients with dry age-related macular degeneration. Dry AMD is the most common…
FDA approves first eye drop for presbyopia
AbbVie (NYSE:ABBV) subsidiary Allergan has received FDA approval for Vuity (pilocarpine HCl ophthalmic solution), a novel eye drop for treating presbyopia. This common age-related condition leads millions of middle-aged people to acquire reading glasses. Vuity is a once-daily prescription eye drop. Presbyopia, or age-related blurry near vision, can be diagnosed through a basic eye exam…
Regenxbio inks deal with AbbVie to pursue development of RGX-314
Regenxbio (NSDQ:RGNX) has closed a license agreement with AbbVie (NYSE:ABBV) to develop RGX-314, a vascular endothelial growth factor inhibitor. The drug has been the focus of Phase 2 and Phase 3 trials exploring its potential in wet age-related macular degeneration (AMD) and diabetic retinopathy. AbbVie will make an upfront payment to Regenxbio of $370 million…
Coherus files BLA for Lucentis biosimilar
Coherus BioSciences (NSDQ:CHRS) has announced that FDA has accepted for review the 351(k) Biologics License Application (BLA) for CHS-201, a biosimilar candidate referencing the blood vessel growth inhibitor Lucentis (ranibizumab). Developed by Genentech, Lucentis first won FDA approval in 2006 to treat wet age-related macular degeneration. Other indications followed, including macular edema following retinal vein…
AGTC announces data from ongoing Phase 1/2 trial
Applied Genetic Technologies Corp. (NSDQ:AGTC) has announced 12-month data from a Phase 1/2 trial involving achromatopsia, an inherited retinal condition affecting roughly 27,000 people in the U.S. and EU. Most people with achromatopsia are legally blind. AGTC is focusing on two gene mutations linked to the disorder known as A3 and B3. The former accounts…
Innovative contact lens delivers glaucoma medication continuously
For nearly half a century, contact lenses have been proposed as a means of ocular drug delivery that may someday replace eye drops, but achieving controlled drug release has been a significant challenge. Researchers at Massachusetts Eye and Ear/Harvard Medical School Department of Ophthalmology, Boston Children’s Hospital, and the Massachusetts Institute of Technology are one…