Glucagon-like peptide-1 (GLP-1) receptor agonists like semaglutide and tirzepatide have cemented their status as two of the most successful drugs in recent memory. Recent projections have estimated that the drug class could fetch $44 billion by 2030 and $71 billion by 2032. But GLP-1 sales could potentially reach greater heights as these therapies move beyond…
Beyond GLP-1: OrsoBio targets mitochondria to treat metabolic disorders from a new and potentially complementary angle
Emerging from stealth in late 2022, Palo Alto–based biopharma startup OrsoBio announced four development programs targeting severe metabolic disorders. The CEO and founder, Dr. Mani Subramanian and chief medical officer and head of development Dr. Rob Myers formerly worked at Gilead. Founded in 2020 with a quest to “try to modulate energy metabolism in severe…
GSK could pay Arrowhead $1B to develop NASH RNAi therapeutic
Arrowhead Pharmaceuticals (NSDQ:ARWR) has signed an exclusive license agreement with GlaxoSmithKline (NYSE:GSK) related to Arrowhead’s experimental RNA interference (RNAi) therapeutic ARO-HSD. The drug is now the subject of a Phase 1/2 study focusing on the drug’s potential as a treatment for nonalcoholic steatohepatitis (NASH), which involves liver inflammation and can cause cirrhosis and liver failure.…
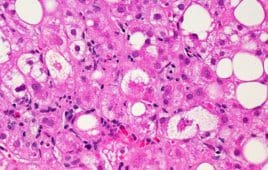
The promise of mitochondria-based therapeutics for NASH
Although nonalcoholic steatohepatitis (NASH) is one of the most common types of chronic liver disease in the U.S., it is a disease that is unfamiliar to many Americans. Despite this, its prevalence is increasing, and by some estimates, 27 million Americans will be living with NASH by 2030. NASH is a progressive condition that starts…
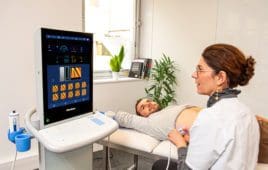
Noninvasive liver exam plays role in fighting viral hepatitis and fatty liver disease
Millions of Americans who are living with hepatitis C virus (HCV) will develop a chronic infection that, if left untreated, can cause serious health problems, including liver disease, cirrhosis, liver failure and liver cancer. More than one-third of HCV-infected individuals progress to advanced fibrosis and cirrhosis, and among those with cirrhosis, about 3–5% per year…
Ben Carson joins Galectin Therapeutics as ‘special consultant’
Former HUD Secretary Dr. Ben Carson, Sr. will assist Galectin Therapeutics Inc. (NSDQ: GALT) with the development of its galectin-3 inhibitor, belapectin. The drug is a potential treatment for nonalcoholic steatohepatitis (NASH), which is also known as nonalcoholic fatty liver disease. Galectin Therapeutics has an ongoing Phase 2b/3 trial to investigate belapectin’s potential in treating cirrhosis…