The latest crop of glucagon-like peptide-1 (GLP-1) agonists, such as semaglutide and tirzepatide (technically, a dual GIP/GLP-1 receptor agonist), have upended the way we treat metabolic disorders, including obesity. These drugs mimic gut hormones, improving blood sugar control and often leading to significant weight loss. But as Kyasha Sri Ranjan, Ph.D., engagement manager at Lifescience…
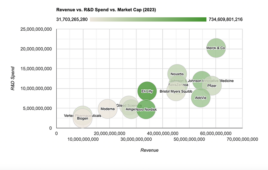
When size doesn’t matter: How Lilly and Novo Nordisk are outperforming pharma giants in value creation
The graphic above shows darker bubbles for companies with larger market caps as of March 25, 2024. Revenue and R&D spending figures are in USD. More data are available when hovering over a given company. After a COVID-19 rebound in public perception and willingness to explore novel modalities, the pharma industry faces mounting pressures…
Amidst empty labs, signs of biotech’s resurgence emerge
In 2023, a year of accelerated regulatory success, a significant number of biotech labs sat empty in major hubs like San Francisco and Boston. The FDA approved 55 novel therapies in 2023, including Leqembi for early Alzheimer’s and Zurzuvae for postpartum depression. The approval number marked the second highest count in three decades (see graph…
A glimpse at Big Pharma’s upper echelon in the first three quarters of 2023
In 2023, demand for GLP-1 receptor agonists such as Lilly’s tirzepatitde and Novo Nordisk’s semaglutide surged just as demand for COVID-19 therapies waned. As a result, Novo Nordisk had 33% growth at constant exchange rates over the first nine months of the year. Similarly, Lilly experienced a 37% jump in revenue in the third quarter,…