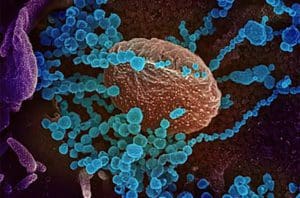
This colorized scanning electron microscope image shows SARS-CoV-2 (round blue objects), the virus that causes COVID-19, emerging from the surface of cells cultured in the lab. [Image courtesy of National Institute of Allergy and Infectious Diseases]
The agency’s reasoning for the decision is grounded in lab data that found the antibody cocktail is not effective against P.1 (Brazilian) or B.1.351 (South African) variants. Those variants comprise more than 10% of COVID-19 infections in the eight states mentioned above.
HHS still recommends Regeneron’s (NSDQ:REGN) REGEN-COV antibody cocktail in areas with significant spread of the P.1 and B.1.351 variants.
Earlier this year, FDA withdrew the EUA for Lilly’s bamlanivimab but continued to authorize its use when paired with etesevimab. The reasoning for doing so was that bamlanivimab alone was ineffective against some SARS-CoV-2 variants.
Filed Under: Infectious Disease



