Note: This feature on the best-selling pharmaceuticals of 2023 was updated on March 27. Changes are possible as more data become available. Sales of drugs with more than one developer are added together (as in the case of the COVID-19 vaccine Comirnaty) unless one of those companies records the net sales for it, i.e., Dupixent.…
The top 100 cell and gene therapy companies to watch in 2023
The cell and gene therapy sector is poised to deliver a wave of new therapies with the potential to cure rare and common diseases. As many as 13 new cell or gene therapies could be approved for use in the U.S., Europe, or both by the end of 2023. While manufacturing and regulatory challenges remain,…
4 top drug discovery innovations of 2022
Late last year, the Galien Foundation highlighted several drug discovery innovations in its annual Prix Galien USA Award Winners, which specifically highlighted drugs from Regeneron and Amgen as well as a platform from Exscientia and the incubators BioLabs and LabCentral. The foundation recently hosted a webinar featuring several executives from the respective winning companies discussing their view…
And the most innovative biotechnology and pharma products are…
The Galien Foundation has selected Regeneron’s (Nasdaq:REGN) Inmazeb (atoltivimab, maftivimab and odesivimab-ebgn) as the best biotechnology product of 2022 and Amgen’s (Nasdaq:AMGN) Lumakras as the best pharmaceutical agent. The organization announced the news last week as part of the Prix Galien USA 2022 awards series in a ceremony in New York City. The antibody cocktail Inmazeb…
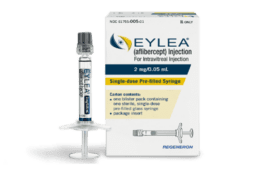
FDA grants priority review of aflibercept in retinopathy of prematurity
Regeneron Pharmaceuticals (Nasdaq:REGN) has announced that the FDA has granted Priority Review of the supplemental Biologics License Application (sBLA) for Eylea (aflibercept) Injection to treat retinopathy of prematurity (ROP) in preterm infants. First approved in 2011 to treat wet age-related macular degeneration, aflibercept is a blockbuster drug. Last year, it generated $9.2 billion for Regeneron…
Regeneron purchases exclusive rights to Libtayo from Sanofi for $900M
Sanofi (Nasdaq:SNY) has agreed to sell Regeneron (Nasdaq:REGN) exclusive international rights of Libtayo (cemiplimab) for $900 million. Sanofi will also receive an 11% royalty on global sales of the drug. Libtayo is a monoclonal antibody targeting the immune checkpoint receptor PD-1 on T-cells. Sanofi could also receive $100 million if the drug hits regulatory milestones…
Gilead Sciences, Merck near the top of Newsweek’s most responsible companies list
A handful of big names in drug discovery and development are among the 500 “most responsible,” according to Newsweek. The outlet published its “America’s Most Responsible Companies 2022” list, marking the third installment of the compilation (in partnership with Statista), this time expanded to include 500 of the largest public corporations around. Companies were judged with an overall…
Early data suggest COVID-19 monoclonal antibodies offer less protection against Omicron variant
The antibody cocktails from Regeneron (NSDQ:REGN) and Eli Lilly (NYSE:LLY) may provide less protection against the Omicron COVID-19 variant than earlier circulating variants. Independent researchers at the Fred Hutchinson Cancer Research Center (Seattle) concluded that bamlanivimab and etesevimab had a decrease in protection against Omicron, according to The Wall Street Journal. Omicron could be more resistant…
REGEN-COV cuts viral load within 7 days in COVID-19 patients
Regeneron Pharmaceuticals (NSDQ:REGN) and Roche (OTCQX: RHHBY) are touting positive results from a Phase 2/3 study investigating the potential of REGEN-COV (casirivimab and imdevimab) antibody cocktail in hospitalized COVID-19 patients. The study met its primary endpoint of reducing viral load within one week in seronegative patients needing low-flow or no supplemental oxygen. REGEN-COV, known as…
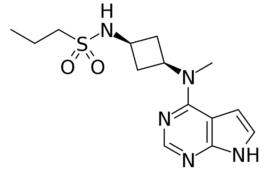
Pfizer’s abrocitinib goes head-to-head with Sanofi’s Dupixent
Pfizer (NYSE: PFE) recently announced that its once-daily oral Janus kinase 1 (JAK1) inhibitor abrocitinib bested Sanofi’s Dupixent (dupilumab) in a Phase 3 study focused on moderate to severe atopic dermatitis (AD). Meanwhile, Sanofi (EPA:SAN) announced that a Dupixent pivotal trial met its primary and secondary endpoints, making it the first biologic to demonstrate significant…
REGEN-COV lowers risk of symptomatic COVID-19 infections by 81% in study
Regeneron’s (NSDQ:REGN) REGEN-COV remains one of the most effective monoclonal antibody therapies in reducing the risk of serious COVID-19 infections and resulting hospitalizations and deaths. A study recently published in the New England Journal of Medicine found that the drug effectively prevented COVID-19 disease in household contacts of individuals infected with the novel coronavirus. When…
Regeneron uncovers genetic mutations that guard against obesity
Regeneron Pharmaceuticals (NSDQ:REGN) has announced that its Regeneron Genetics Center (RGC) has discovered rare genetic mutations associated with protection against obesity. RGC researchers learned that people with at least one inactive copy of the GPR75 gene tend to weigh about 12 pounds less than those without that mutation. In addition, those individuals faced a 54%…
Pandemic and disease burden are ‘existential’ threats, experts argue
While the COVID-19 pandemic has highlighted the prowess of the pharmaceutical industry, it should also “serve as a major wake-up call,” said Dr. George Yancopoulos, co-founder, president and CSO of Regeneron (NSDQ:REGN), at a panel at the virtual USA India Chamber of Commerce meeting. COVID-19 has underscored the importance of preparing for infectious pandemics and…
FDA authorizes lower dose of REGEN-COV COVID-19 antibody cocktail
Regeneron (NSDQ:REGN) has announced that the FDA has signed off on a 1,200 mg subcutaneous or intravenous dose of its REGEN-COV antibody cocktail. The quantity is half of the initially authorized dose. The agency had previously authorized a 2,400 mg dose of the vaccine, including a combined dose of 1,200 mg of Casirivimab and 1,200…
HHS pauses use of Lilly’s COVID-19 antibody cocktail in several states
The U.S. Department of Health and Human Services (HHS) has recommended pausing the combination of Eli Lilly’s (NYSE:LLY) bamlanivimab/etesevimab in eight states, including Illinois, Massachusetts, Arizona, California, Florida, Indiana, Oregon and Washington. The agency’s reasoning for the decision is grounded in lab data that found the antibody cocktail is not effective against P.1 (Brazilian) or B.1.351…
Regeneron exec shares how Ebola inspired COVID-19 treatment
The speed that pharmaceutical companies developed COVID-19 treatments and vaccines was “seemingly miraculous,” said Dr. George Yancopoulos, co-founder of Regeneron (NSDQ: REGN), in remarks when receiving a Titans of Global Health award. “But these miracles were actually born many decades earlier,” he said at the virtual event ceremony from the American Friends of the Hebrew University.…
Roche retools COVID-19 strategy
The Swiss pharma giant Roche (OTCMKTS:RHHBY) has canceled two Phase 2 COVID-19 studies while looking to identify a new site to conduct a clinical study for the oral antiviral AT-527, which it is developing with Atea Pharmaceuticals (NSDQ:AVIR.O). The two companies were looking to launch a trial in the U.K., but falling COVID-19 cases there have…
Regeneron touts 70% death risk reduction in COVID-19 antibody drug
Regeneron Pharmaceuticals (NSDQ:REGN) announced today that its COVID-19 antibody drug reduced hospitalization and death risk by 70% in clinical trials. The definitive Phase 3 outcomes trial in high-risk, non-hospitalized COVID-19 patients met its primary endpoint in displaying that the REGEN-COV (casirivimab with imdevimab) significantly reduced the risk of hospitalization or death by 70% (1,200 mg…
11 COVID-19 therapies you need to know
The pharmaceutical industry launched a herculean effort to battle the COVID-19 pandemic early in 2020, developing an array of vaccines and other therapeutics while also identifying existing drugs suitable for treating patients sick with severe coronavirus infections. FDA recently granted emergency authorization to two SARS-CoV-2 vaccines and several other treatments. Here are 11 of the…
Regeneron antibody cocktail wins emergency use authorization
Regeneron Pharmaceuticals (NSDQ:REGN) has announced that the FDA has granted emergency use authorization (EUA) for the combination of casirivimab and imdevimab to treat COVID-19. The monoclonal antibody cocktail is reportedly the first treatment to show “statistically significant anti-viral activity” against COVID-19, according to its developer. In a trial, the combination of drugs cut hospitalization or emergency…
Guess who the pharma industry is supporting for president
The pharma industry has put its weight behind Democratic presidential candidate Joe Biden, reversing a longstanding fundraising trend that has favored the GOP. Republican candidates have received 64% of pharma industry contributions since 1990, according to the Center for Responsive Politics’ Open Secrets. But the industry is shifting its support to Democrats in 2020. So far,…
FDA approves first Ebola virus treatment
The FDA granted approval to Inmazep, a mixture of three monoclonal antibodies, making it the first FDA-approved treatment for Ebola virus. Regeneron Pharmaceuticals (NSDQ:REGN) received the approval, having recently been in the news for providing its investigational COVID-19 therapeutic to President Donald Trump. Inmazeb targets the glycoprotein that is on the surface of the Ebola virus.…
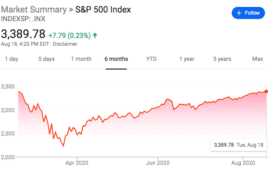
Medtech stocks help boost S&P rally
Medtech stocks helped the S&P 500 Index reach record highs today amid a bounceback from the lows of the COVID-19 pandemic. Reuters reported that medtech played its part in the record turn of the market, with Abiomed (NSDQ:ABMD), Regeneron Pharmaceuticals (NSDQ:REGN) and West Pharmaceutical Services (NYSE:WST), all of which are involved in developing COVID-19 therapeutics, all rising more than 50% since the index’s…
Researchers identify potent antibody cocktail to treat COVID-19
Researchers at the University of Maryland School of Medicine (UMSOM) said they identified a promising anti-viral cocktail therapy against COVID-19. According to a news release, the researchers evaluated several human antibodies to determine the most potent combination to be mixed and used against SARS-CoV-2, the virus causing COVID-19. In collaboration with Regeneron Pharmaceuticals (NSDQ:REGN), UMSOM published…
Regeneron launches clinical trial of COVID-19 antibody ‘cocktail’
Regeneron Pharmaceuticals (NSDQ:REGN) announced today that it initiated the first clinical trial of its dual antibody cocktail for preventing COVID-19. The REGN-COV2 clinical program will include populations of hospitalized COVID-19 patients, non-hospitalized symptomatic COVID-19 patients, uninfected people in groups that have a high risk of exposure (healthcare workers or first responders, for example) and uninfected people…