Pulmonary arterial hypertension (PAH), a rare, progressive and life-threatening blood vessel disorder, affects some 500 to 1,000 new patients each year in the U.S. FDA recently approved Opsynvi, a first-of-its-kind once-daily single-tablet combination therapy from Johnson & Johnson. “With this approval, our portfolio now includes treatments that address all three guideline-recommended pathways,” said a J&J…
Could Wegovy’s cardiovascular label expansion be a catalyst for GLP-1 obesity drug coverage?
The recent FDA approval of a cardiovascular risk reduction indication for Wegovy (semaglutide) could point toward a significant opportunity for pharma companies seeking to reshape payer perceptions and expand coverage for next-gen metabolic therapies. This regulatory shift, allowing Wegovy to be prescribed for reducing the risk of major adverse cardiovascular events such as heart attack…
Fixed-dose macitentan-tadalafil polypill outperforms monotherapies in PAH
Pulmonary arterial hypertension (PAH) is a relatively rare disorder, affecting roughly 15 to 50 people per million within the U.S. and Europe. Treating it with a single therapy can be challenging, and medication adherence often presents a common hurdle for the condition. Combination therapy has thus become a widely used option in the treatment of…
Can AI ‘move fast and cure things’ in healthcare?
The entry of AI into healthcare is starkly different from the accepted adage of “move fast and break things” in consumerism. In episode 1 of AI Meets Life Sci, Kayleen Brown, managing editor at DeviceTalks, and Brian Buntz, pharma and biotech editor, discuss the opportunities, limitations, and direct impact of Ai in healthcare fields including…
Prix Galien Awards: The most innovative biotech, pharma, and orphan drugs of 2023
Prix Galien names 2023 winners in pharma and biotech In the world of medical innovation, few accolades carry as much weight as the Prix Galien Awards, which highlight the advances in biotech, pharmaceuticals, and other domains. The 2023 winners include Bristol Myers Squibb’s Camzyos (mavacamten) as the best biotechnology product and Lilly’s Mounjaro (tirzepatide) and…
Will GLP-1 drugs transition from obesity and diabetes to diverse clinical indications?
The explosive sales growth of GLP-1 drugs has analysts projecting that the antiobesity drugs could be a $44 billion market by 2030. Some observers are more upbeat, projecting that the sector could be worth more than $100 billion in the coming years. Pfizer CEO Albert Bourla projects that the market will reach $90 billion by…
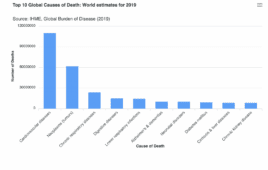
Data-driven insights into the leading causes of death, including cardiovascular disease and cancer
Data sourced from Our World in Data Cardiovascular disease and cancer remain the leading causes of death in the world, based on an analysis of data sources ranging from World Health Organization and Our World in Data. While respiratory illnesses were the third most common cause of death in 2019, deaths in this category…
Anti-obesity drugs to command a $44 billion market by 2030
As obesity rates continue to soar across the globe, analysts anticipate that the market for anti-obesity drugs will skyrocket in the coming years. Goldman Sachs projects the market to be worth $44 billion by 2030. That’s an almost 16-fold expansion from its valuation of approximately $2.82 billion in 2022. Barclays is even more upbeat on…
New study sheds light on Eliquis and Xarelto switching
For patients with an elevated stroke risk, switching anticoagulant medications could be a health gamble. Those who change from Eliquis (apixaban) to Xarelto (rivaroxaban) could face almost double the risk of stroke or severe bleeding. Conversely, sticking with Eliquis or transitioning from rivaroxaban to Eliquis appears to be a safer option. Those are key takeaways…
‘Long vax’ phenomenon gets closer look in recent studies
In 2020, researchers witnessed the emergence of post-acute sequelae of SARS-CoV-2 (PASC) — more commonly referred to as “long COVID.” Now, the notion of “long vax,” persistent and varying symptoms following COVID-19 vaccination, has come into focus, as Science has noted. This phenomenon, while not as widespread as long COVID, has concerned some in the…
Lilly publishes promising phase 2 trial data for retatrutide, a potential obesity therapy
Lilly has announced promising data from its NCT 04881760 phase 2 study of retatrutide, a potential obesity drug. The drug candidate, a single peptide with agonist activity at the glucose-dependent insulinotropic polypeptide (GIP), GLP-1 and glucagon receptors, was associated with significant weight loss, improved glycemic control and cardiovascular health. At 24 weeks, retatrutide (1 mg,…
AstraZeneca launches pilot program for Cordio Medical’s AI heart failure app
AstraZeneca and the Bellvitge University Hospital plan to launch the first pilot program for the Cordio Medical HearO app. The HearO smartphone app provides widespread access to medical-grade technology for patients with congestive heart failure (CHF). It enables the patient to monitor symptoms, manage treatment and improve quality of life. Astrazeneca said the pilot program aims to…
FDA approves expanded use of Farxiga (dapagliflozin) for heart failure patients regardless of ejection fraction status
FDA has approved an expanded indication for AstraZeneca’s Farxiga (dapagliflozin). This decision, marking a significant milestone in Farxiga’s development, opens up its use in patients with heart failure, regardless of their ejection fraction status. This brings the total number of Farxiga’s approved indications to five. Ejection fraction in heart failure Ejection fraction is a key measure…
Tirzepatide versus semaglutide: Which contender will prevail in the battle against obesity and type 2 diabetes?
Eli Lilly‘s (NYSE:LLY) tirzepatide achieved up to 15.7% weight loss in the SURMOUNT-2 study, sparking a potential tirzepatide versus semaglutide competition in the obesity and type 2 diabetes treatment markets. The phase 3 study enrolled 938 participants with diverse backgrounds. Tirzepatide promises to be a megablockbuster with a number of analysts pegging peak annual sales…
The 50 best-selling pharmaceuticals of 2022: COVID-19 vaccines poised to take a step back
The COVID-19 pandemic has had a profound impact on the best-selling pharmaceuticals, leading to shifts in the list with Pfizer and BioNTech’s Comirnaty surpassing AbbVie’s Humira for the No. 1 spot in 2021. That momentum continued in 2022, with Pfizer and BioNTech jointly raking in $59.1 billion in revenue from the sales of the COVID-19…
The next wave: 10 promising investigational antihypertensive drugs to watch
Cardiovascular diseases remain a leading cause of morbidity and mortality worldwide. This review highlights ten promising antihypertensive drug candidates with the potential to address pulmonary arterial hypertension (PAH), resistant hypertension and uncontrolled hypertension. Aprocitentan, developed by Janssen NYSE:JNJ) and Idorsia (SIX:IDIA), is an experimental oral drug designed to treat resistant hypertension. As a dual endothelin…
BPIFB4 gene therapy could protect against heart aging
A recently published paper in Cardiovascular Research found that a gene frequently found in centenarians could safeguard heart functionality. In a rodent model, a team of researchers found that the bactericidal/permeability-increasing fold-containing family-B member-4 gene (BPIFB4) gene protected against deterioration of heart function in middle-aged mice (14 months old). The researchers, led by Professor Paolo Madeddu of the University…
Lilly gets FDA review for empagliflozin in chronic kidney disease
The FDA has accepted a supplemental New Drug Application (sNDA) for Jardiance (empagliflozin) tablets as a potential therapy to reduce the risk of kidney disease progression and cardiovascular death in adults with chronic kidney disease (CKD). Some 37 million people in the U.S. have CKD. Lilly (NYSE:LLY) is developing the drug with Boehringer Ingelheim. The…
CinCor stock surges after AstraZeneca announces plan to buy company for up to $1.8B
AstraZeneca (LON:AZN) announced it had reached an agreement to acquire CinCor Pharma (Nasdaq:CINC). After announcing the proposed deal, CinCor’s share price jumped 144% to $28.74. AstraZeneca has agreed to a tender offer to acquire outstanding shares of CinCor for $26 per share, or $1.3 billion. CinCor specializes in developing therapies for hypertension and chronic kidney…
CardiaCare to partner with Dr. Reddy’s Laboratories in India
CardiaCare recently announced it signed a strategic licensing agreement with Dr. Reddy’s Laboratories to further develop CardiaCare’s wearable atrial fibrillation treatment device. Through the agreement, Dr. Reddy’s Laboratories will lead necessary clinical studies for CardiaCare to receive regulatory clearance in India. CardiaCare is developing a non-invasive, personalized neuromodulation wearable for atrial fibrillation treatment. It will…
CinCor’s COO has a mission to transform hypertension treatment
The clinical-stage biopharma CinCor is developing baxdrostat, a highly selective, oral small molecule inhibitor of aldosterone synthase, for hypertension. Catherine Pearce, chief operating officer and co-founder of CinCor, acknowledges that the company has received criticism for its plan to bring a new drug into the highly genericized blood pressure control market. “The reality is that…
J&J’s aprocitentan shows promise in difficult-to-control hypertension
Johnson & Johnson (NYSE:JNJ) has announced that the investigational antihypertensive drug aprocitentan significantly lowered blood pressure (BP) when used in conjunction with background antihypertensive therapy in the Phase 3 PRECISION study. Sharing the results in collaboration with Idorsia (OTCMKTS:IDRSF), J&J noted that the drug candidate helped maintain a reduction in blood pressure for 48 weeks. …
European and Canadian authorities move to limit risk from JAK inhibitors
The European Medicines Agency (EMA) is backing a series of measures to reduce the risk of serious side effects associated with Janus kinase (JAK) inhibitors, limiting their use in high-risk individuals. Similarly, Health Canada is recommending updated labeling for the drug class to disclose the risk of serious cardiovascular events and cancer. The agency is…
Lilly wins FDA Fast Track designation for tirzepatide in patients with weight-related comorbidities
Eli Lilly (NYSE:LLY) has received Fast Track designation from FDA to investigate tirzepatide in obese or overweight adults with weight-related comorbidities. The Fast Track status is designed to compress the time needed for FDA to approve tirzepatide for use in adults with obesity or overweight with weight-related comorbidities. The drug is a glucose-dependent insulinotropic polypeptide…
Why Algernon is investigating DMT in stroke rehabilitation
The clinical-stage company Algernon Pharmaceuticals (CSE:AGN; Frankfurt:AGW0; OTCQB: AGNPF) has received approval to run a Phase 1 clinical study of an IV formulation of AP-188. The study will explore the drug candidate’s potential, a formulation of the classic psychedelic N,N-dimethyl tryptamine or DMT, to treat stroke patients in the Netherlands. The Stichting Beoordeling Ethiek Biomedisch…