 Candel Therapeutics (NSDQ:CADL) recently announced that it had entered a strategic collaboration with microrobotics startup Bionaut Labs (Los Angeles) to explore precision-targeted delivery of oncolytic viral immunotherapies.
Candel Therapeutics (NSDQ:CADL) recently announced that it had entered a strategic collaboration with microrobotics startup Bionaut Labs (Los Angeles) to explore precision-targeted delivery of oncolytic viral immunotherapies.
To learn more about the company, we reached out to Candel’s Dr. Paul Peter Tak, president and CEO of the company.
Candel Therapeutics has two viral immunotherapy platforms, one based on adenovirus and another on herpes simplex virus (HSV). “Both of which are designed to be locally administered, have direct cancer-killing activity and elicit both local and systemic anti-tumor immune response,” Tak said.
The company believes these viral immunotherapies induce immune cells that target patients’ specific tumor antigens. As a result, such therapies could potentially “improve responses in immunologically ‘hot’ tumors while at the same time infiltrating the tumor microenvironment, transforming non-inflamed “cold” tumors with limited immune response into ‘hot’ tumors,” Tak said.
CAN-2409 image from Candel Therapeutics
The first of the company’s immunotherapy platforms, CAN-2409, is built on Candel’s adenovirus platform. “CAN-3110 and all of our discovery programs are built around our HSV platform, which has a number of key advantages, including the flexibility to design constructs that are either replicating or non-replicating, as well as introducing very large payload sizes,” Tak said.
In the following Q&A, Tak sheds more details on CAN-2409 and CAN-3110 in light of the partnership with Bionaut.
Drug Discovery & Development (DDD): Can you share more about CAN-2409 and CAN-3110?

Dr. Paul Peter Tak. Image courtesy of Candel Therapeutics.
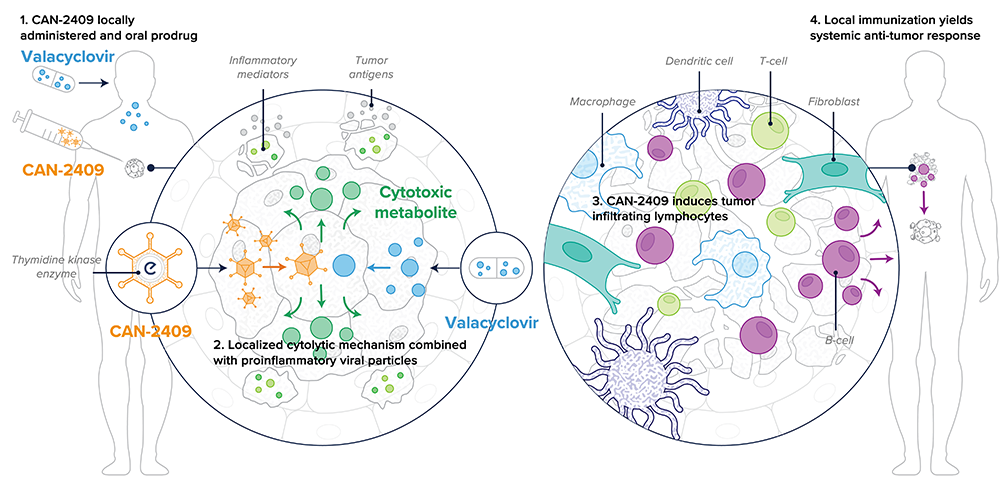
Dr. Paul Peter Tak: CAN-2409, Candel’s most advanced oncolytic viral immunotherapy candidate, is a replication-deficient adenovirus that delivers the herpes simplex virus thymidine kinase (HSV-tk) gene to cancer cells. HSV-tk is an enzyme that locally converts orally administered valacyclovir into a toxic metabolite that kills nearby cancer cells. The intra-tumoral administration results in the release of tumor-specific neoantigens in the microenvironment. At the same time, the adenoviral serotype 5 capsid protein elicits a strong pro-inflammatory signal in the tumor microenvironment. This creates the optimal conditions to induce a specific CD8+ T cell-mediated response against the injected tumor and uninjected distant metastases for broad anti-tumor activity.
DDD: What potential indications do you foresee for CAN-2409?
Tak: Because of its versatility, CAN-2409 has the potential to treat a broad range of solid tumors. Monotherapy activity, as well as combination activity with standard of care radiotherapy, surgery, chemotherapy and immune checkpoint inhibitors, have previously been shown in several preclinical and clinical settings. Furthermore, CAN-2409 presents a favorable tolerability profile; more than 700 patients have been dosed to date, supporting the potential for combination with other therapeutic strategies without inordinate concern of overlapping adverse events. Currently, Candel is evaluating the effects of treatment with CAN-2409 in non-small cell lung cancer, high-grade glioma, pancreatic cancer, and localized, non-metastatic prostate cancer in ongoing Phase 2 and Phase 3 clinical trials.
DDD: What about CAN-3110?
Tak: CAN-3110 is an engineered oncolytic HSV where the expression of ICP34.5, the gene responsible for viral replication, has been placed under the control of a tumor-specific Nestin promoter. This modification of the viral genome enables us to maintain the function of ICP34.5. This HSV protein allows virus replication even in the presence of a suppressive interferon response, under strict control and only in tumor cells.
ICP34.5 is often deleted in other HSV oncolytic viruses that may be less tumor-selective with an intent of achieving a favorable safety profile, but this often results in weak viruses characterized by poor replication ability and an ability to generate a limited immune response.
Nestin is a cytoskeletal protein that is overexpressed in glioma cells, but it is absent in the healthy adult brain. In CAN-3110, ICP34.5 expression is controlled by the Nestin promoter enabling viral replication selectively in tumor cells. This replication-competent HSV construct provides tumor-specific cytolytic activity in animal models while sparing healthy cells.
Candel is evaluating the effects of treatment with CAN-3110 for recurrent glioblastoma in an ongoing Phase 1 clinical trial.
Filed Under: clinical trials, Drug Discovery, Drug Discovery and Development, Oncology



