Protein misfolding image courtesy of Gain Therapeutics
Over the last decade, drugs that bind to allosteric sites have emerged as an attractive approach in small molecule drug design, offering new possibilities for targeting various diseases, including conditions historically known as “undruggable”. Unlike traditional small molecules that bind to a target protein’s endogenous orthosteric or active site, allosteric small molecules selectively bind and modulate protein function at a distant location on the protein’s surface. This unique mechanism allows for precise and selective control of protein activity, offering potential advantages over conventional small-molecule medicines.
While allosteric activation and inhibition have proven their clinical utility, scientists are unearthing new methods of developing allosteric therapeutics that can better modulate protein function as determined by disease pathology. Furthermore, using computational and physics-based approaches allows drug developers opportunities to discover novel binding sites based on 3D protein structures. Together, the next generation of allosteric small molecules hold the promise of being more powerful and versatile, potentially leading to safer, more effective compounds and better outcomes for patients.
Advantages and disadvantages of current allosteric modulation
The first advantage of allosteric drugs is their ability to fine-tune protein function. While orthosteric drugs compete with the natural substrate, binding to the allosteric site can inhibit the target protein’s activity in a non-competitive manner, but it can also enhance the protein’s function without interfering with endogenous substrate binding, depending on the therapeutic goal.
This level of non-competitive modulation provides greater control over complex biological processes, such as cellular localization or interaction with other biological components. Further, compared to orthosteric drugs, allosteric drugs can exhibit a higher degree of selectivity because the allosteric sites are quite unique, thus providing selectivity even across closely related proteins, which minimizes the potential for off-target effects and reduces the risk of unwanted clinical adverse events while enabling a more targeted and personalized approach to treatment. Yet another reason to target allosteric sites is that, in many cases, allosteric binding is the only way to affect the activity of a protein that is “undruggable” due to the inability to target the orthosteric site. Finally, when dealing with diseases where resistance emerge, such as cancer and infectious diseases, allosteric drugs are quite effective against mutations that have emerged in response to treatment with orthosteric drugs. In this scenario, allosteric and orthosteric drugs can be given in combination. Since they are acting on different sites, no single mutation can confer resistance to both treatments, thus preventing disease progression through resistance.
Positive and negative
In general, currently available allosteric drugs fall into two categories: positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs). This current generation of PAMs and NAMs alters the endogenous substrate’s binding ability to the active site through allosteric activation or inhibition, respectively (see Figure 1). These modulators were serendipitously discovered through conventional enzymatic or functional assays originally designed for identifying orthosteric ligands. However, a rational, systematic method for uncovering allosteric ligands, particularly those exerting non-standard modulation, is currently lacking.
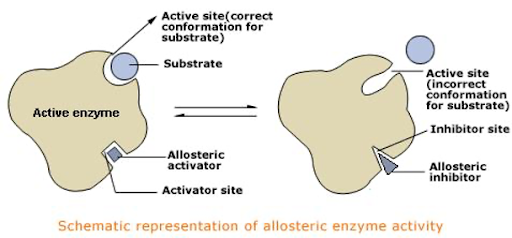
Figure 1: This visual representation of allosteric enzyme activity shows the distinct mechanisms of positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs) in influencing the binding ability of the endogenous substrate.
A new generation of allosteric drugs
While allosteric activation or inhibition provides clinically-relevant modulation of diseased proteins, it is clear that protein biology is more complex, necessitating a new way of developing allosteric therapeutics. Today, scientists are developing a new wave of allosteric drugs that allow for the fine-tuning of protein activity by changing the protein folding itself, enabling the modulation of specific disease-related conformations or interactions while preserving normal physiological functions.
Beyond activation and inhibition, this next generation of allosteric modulators offers protein stabilization, destabilization, and degradation. This control level creates novel therapeutic opportunities with the potential to achieve greater efficacy and expanding the so-called “druggable genome” up to 90%. Additionally, these novel allosteric modulators may even bind ‘silently’, meaning that they give no signal in a biochemical assay, but can change the protein function within the cell’s environment.
One disease indication that can benefit from this next generation of allosteric therapies is GBA-associated Parkinson’s disease. The GBA-1 genetic mutation is the most significant genetic risk factor for developing PD and affects approximately 15% of the patient population. The mutation causes dysfunctional misfolding of the lysosomal enzyme glucocerebrosidase (GCase) and the subsequent accumulation of alpha-synuclein, a hallmark in PD, and subsequent neuronal death.
In preclinical studies, the allosteric modulator GT-02287, which is currently in development by Gain Therapeutics, prevents misfolding of the GCase protein, enabling the enzyme to function properly and restore lysosomal health, ultimately preventing alpha-synuclein aggregation and neuronal cell death. This example demonstrates how the next generation of allosteric modulators could provide transformative, disease-modifying activity for this population of PD patients and potentially for other neurodegenerative diseases characterized by GCase dysfunction or buildup of alpha-synuclein.
Addressing challenges with computational and physics-based technologies
Despite their immense potential, the development of allosteric drugs presents several challenges. Identifying suitable allosteric sites within target proteins and designing molecules that can bind to these sites with high affinity and selectivity remains a complex task. However, advancements in computational modeling of the protein’s 3D conformations, grounded in fundamental physical models, can uncover druggable allosteric sites that were previously missed. The same methods, combined with machine learning and other data mining tools, enable researchers to screen huge collections of virtual compounds, enabling the discovery and optimization of allosteric drugs.
By combining these advanced tools, we can identify and design molecules that selectively bind to allosteric sites with high affinity and specificity, ultimately leading to the development of innovative and effective allosteric drugs for treating such complex diseases.
As our understanding of protein allosteric regulation continues to deepen, and as more allosteric drugs enter clinical trials, this unique small molecule approach will continue to revolutionize drug discovery and lead to innovative therapies for a wide range of diseases.
With more than two decades of experience in computational chemistry and drug discovery, Xavier Barril, Ph.D., has served as Chief Scientific Officer at Gain Therapeutics since January 2018. Co-authoring more than 90 papers and 14 patents, Barril is the inventor of the innovative platform technology that Gain Therapeutics is exploiting to identify novel allosteric modulators.
Filed Under: Drug Discovery and Development, machine learning and AI



