 Today, an FDA advisory panel is convening to evaluate the Ad26.COV2.S COVID-19 vaccine from Johnson & Johnson (NYSE:JNJ).
Today, an FDA advisory panel is convening to evaluate the Ad26.COV2.S COVID-19 vaccine from Johnson & Johnson (NYSE:JNJ).
The vaccine has a favorable safety profile and significant efficacy after a single dose.
Here are several themes that are likely to be among the topics discussed in the Vaccines and Related Biological Products Advisory Committee meeting.
1. The J&J vaccine uses an adenovirus vector as opposed to mRNA
In contrast to Moderna and Pfizer’s mRNA vaccines, J&J’s vaccine uses a replication-incompetent recombinant adenovirus type 26 as a vector to express the SARS-CoV-2 spike protein.
The Ad26 platform is not new, having been administered to nearly 200,000 people in other vaccine candidates.
AstraZeneca’s COVID-19 vaccine also uses an adenovirus vector, as does the Rusian vaccine Sputnik V.
2. Efficacy is in the ballpark of 67%
The Moderna and Pfizer two-dose vaccines had efficacy rates in the range of 95% in Phase 3 clinical trials.
While it is often difficult to compare results of separate clinical studies, the J&J’s vaccine efficacy appears to be a good deal lower — 67% overall. Efficacy was higher in the U.S. (72%) but lower in South Africa (57%), where a highly transmissible variant has achieved broad penetration.
It’s worth noting that the primary endpoint of the study was the prevention of moderate to severe disease. That is subtly different from the primary endpoint for the Pfizer-BioNTech, Moderna and AstraZeneca studies, which measured their vaccines’ ability to prevent symptomatic COVID-19.
The J&J vaccine fares better in its ability to prevent severe or critical COVID-19. There, it has overall efficacy of 76.7% and 80.5% in participants between the ages of 18 and 59.
3. Vaccine efficacy against severe and critical disease increased over time
Vaccine efficacy for the J&J vaccine increased steadily after administration, showing a steady increased 56 days after inoculation. Performance of the vaccine was similar across locations, including areas with a high incidence of circulating variants such as South Africa.
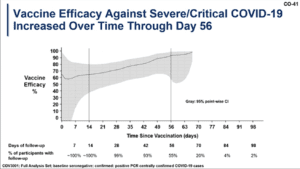
Vaccine efficacy over time for the J&J vaccine.
4. A second dose could boost efficacy
As part of its ENSEMBLE 2 trial, J&J is testing a two-dose regimen of its COVID-19 vaccine. Early data suggests that a second dose boosts the level of neutralizing-antibody titers by a factor of 2.6 to 2.9, “which may well confer more protection,” said vaccine expert Dr. Paul Offit in the VRBPAC meeting. Offit, who is the Vaccine Education Center at Children’s Hospital of Philadelphia, has stressed the importance of the second dose for the mRNA vaccines now available in the U.S.
Early data from non-human primates showed full protection in the lung from a single vaccine dose of the J&J vaccine even four months after administration. The ability of a single dose to provide rapid onset of protection led the company to apply for emergency use authorization for the single-dose regimen.
5. The J&J vaccine could be a powerful tool for ending the pandemic
According to recent research published in Social Science Research Network, a single-dose vaccine with lower efficacy than two-dose vaccines could be a more potent tool in returning society to normalcy. The researchers’ models showed that the single-dose regimen of a vaccine such as J&J’s helps suppress peak infections and cumulative cases more effectively. The models assumed that two-dose vaccines, though ultimately more effective, build up immunity more slowly.
Complicating matters, however, is recent data from Public Health Matters in the U.K. suggesting that a single dose of the Pfizer/BioNTech vaccine has an efficacy rate of 72% after three weeks — incrementally higher than the Johnson & Johnson vaccine Phase 3 results. The study from Public Health Matters involved 15,121 healthcare workers who had not previously tested positive for COVID-19.
6. J&J has experience providing similar vaccines to high-risk populations
Johnson & Johnson has used its AdVac platform to vaccinate breastfeeding and pregnant women as well as children within its Ebola program. The company also has experience vaccinating elderly and various populations across continents. Pregnancy could heighten risk of severe illness from COVID-19.
7. The Phase 3 trial was large
Involving 43,325 randomized participants, the J&J Phase 3 clinical trial was about as large as that Pfizer and BioNTech, which had 43,538 participants in November. As of Nov. 20, 38,955 of the Pfizer-BioNtech participants had received two doses of the vaccine. The Moderna trial involved 30,420 participants.
8. Authorization likely happen soon
Given the favorable clinical trial data and the current vaccine shortage, FDA is likely to allow emergency use authorization of the vaccine in the coming days. Although the efficacy of the single-dose vaccine trails that of the two authorized mRNA vaccines, it is considerably higher than FDA’s efficacy requirement of at least 50%. Furthermore, the vaccine candidate is among the first to have clinical trial data from South Africa and Latin America, two regions with a substantial presence of highly infectious variants.
Other factors weighing in J&J’s favor are the vaccine’s storage requirements (only requiring refrigeration) and its relative ease of administration as a single-dose vaccine.
The Ad26.COV2.S was also well tolerated by the majority of clinical trial participants. Most local adverse events were Grade 1 or Grade 2 and were transient. Grade 3 events were infrequent. However, solicited adverse event rates for fever (8.4%), fatigue (16.7%), headache (15.2%) and muscle pain (20.5%) were higher than they were for currently authorized mRNA vaccines.
A numerical imbalance was observed for thrombotic and thromboembolic events with 14 vaccine recipients having venous thromboembolic events such as deep vein thrombosis or arterial thromboembolic events. A total of 10 placebo recipients had such events .
Filed Under: clinical trials, Drug Discovery, Drug Discovery and Development, Infectious Disease



