Novo Nordisk has received a considerable amount of attention for its meteoric growth of late, but Eli Lilly isn’t far off in that domain as of late. Lilly recently topped the S&P 500 healthcare index in terms of growth, jumping almost 34% as demand for its metabolic therapies surge. Last year, Lilly projected it would…
Best-selling pharmaceuticals of 2023 reveal a shift in pharma landscape
Note: This feature on the best-selling pharmaceuticals of 2023 was updated on March 27. Changes are possible as more data become available. Sales of drugs with more than one developer are added together (as in the case of the COVID-19 vaccine Comirnaty) unless one of those companies records the net sales for it, i.e., Dupixent.…
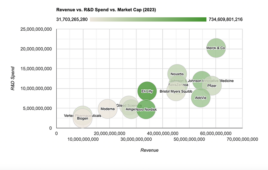
When size doesn’t matter: How Lilly and Novo Nordisk are outperforming pharma giants in value creation
The graphic above shows darker bubbles for companies with larger market caps as of March 25, 2024. Revenue and R&D spending figures are in USD. More data are available when hovering over a given company. After a COVID-19 rebound in public perception and willingness to explore novel modalities, the pharma industry faces mounting pressures…
Beyond GLP-1: OrsoBio targets mitochondria to treat metabolic disorders from a new and potentially complementary angle
Emerging from stealth in late 2022, Palo Alto–based biopharma startup OrsoBio announced four development programs targeting severe metabolic disorders. The CEO and founder, Dr. Mani Subramanian and chief medical officer and head of development Dr. Rob Myers formerly worked at Gilead. Founded in 2020 with a quest to “try to modulate energy metabolism in severe…
Pfizer accuses former employees of trade secret theft
Pfizer (NYSE:PFE) is suing Regor Therapeutics, a China-headquartered company that announced a $1.5 billion diabetes alliance with Eli Lilly (NYSE:LLY) in late 2021. Filed in the U.S. District Court for the District of Delaware, Pfizer’s lawsuit also accuses former employees Xiayang Qiu, Min Zhong and 10 unnamed individuals of using its trade secrets as the…
Novo Nordisk to test oral semaglutide as an obesity therapy
Global pharma firm Novo Nordisk (NYSE:NVO) will launch a Phase 3a study to investigate the potential of oral Ozempic (semaglutide) to treat obesity. The drug is currently indicated in a subcutaneous form in the U.S. for people with type 2 diabetes to improve glycemic control in conjunction with diet and exercise and to lower diabetics’…
FDA sends Novo Nordisk refusal to file letter for weekly 2-mg semaglutide
Novo Nordisk announced that the FDA issued a refusal-to-file letter related to its application to expand the label for once-weekly subcutaneous Ozempic (semaglutide). The company had sought to expand the label for a 2.0-mg dose of once-weekly semaglutide to treat type 2 diabetes. It filed the letter on Jan. 20, 2021. The letter indicates that…
Vertex Pharmaceuticals wins FDA fast track designation for its cell-based diabetes treatment
Vertex Pharmaceuticals announced today that FDA has granted fast track designation for its VX-880, an investigational human stem cell-derived islet cell therapy for type 1 diabetes. Vertex has begun a clinical trial for VX-880 (formerly known as STx-02) in patients with type 1 diabetes (T1D) with severe hypoglycemia and impaired hypoglycemia awareness. “Ours is the only…