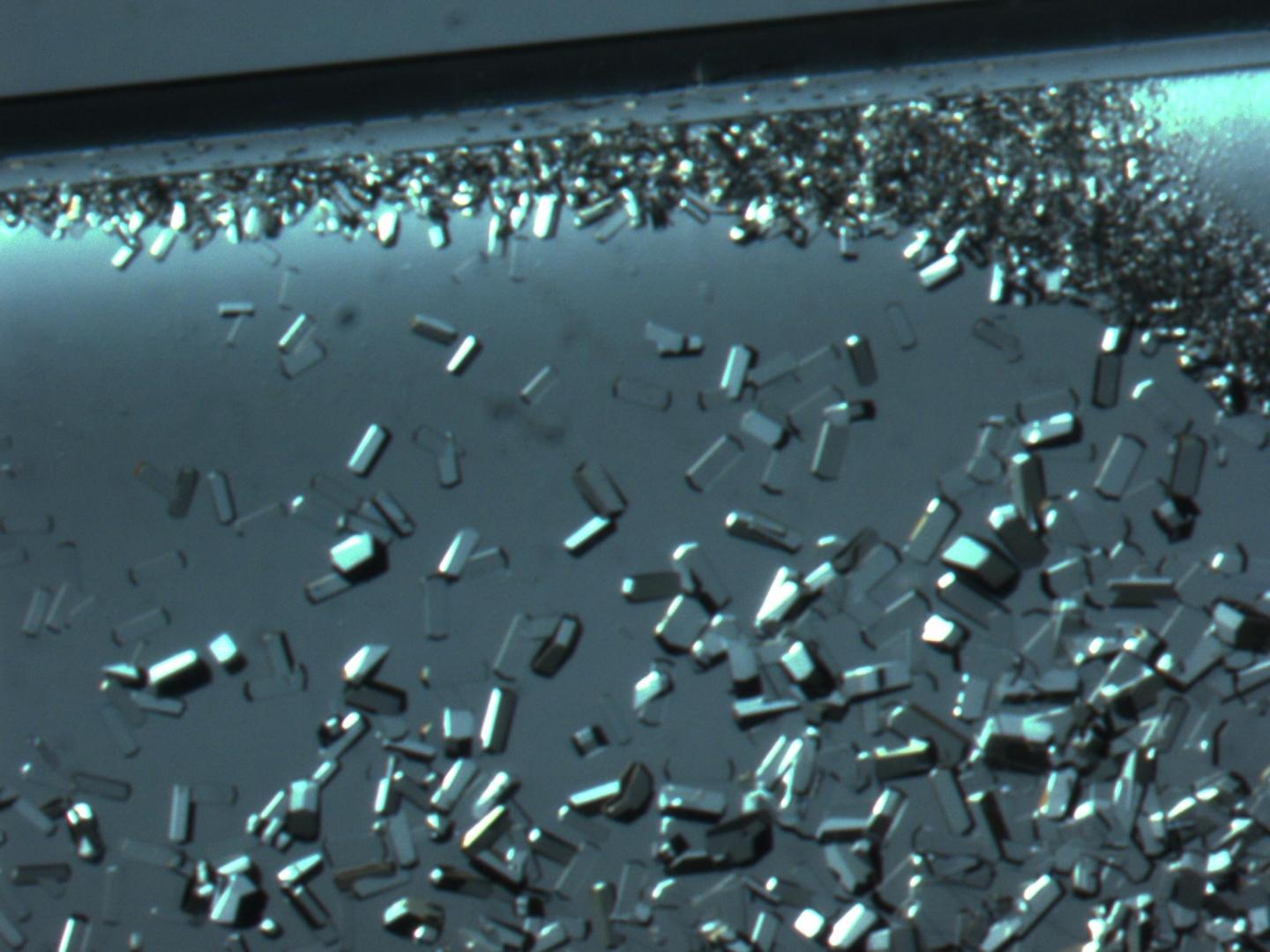
This is a Lysozyme Crystal formation as seen under a light microscope. Crystals grown in microgravity typically reflect fewer imperfections, making them more ideal for drug development and other research. Source: Lawrence DeLucas
Crew members aboard the International Space Station will begin conducting research this week to improve the way we grow crystals on Earth. The information gained from the experiments could speed up the process for drug development, benefiting humans around the world.
Proteins serve an important role within the human body. Without them, the body wouldn’t be able to regulate, repair or protect itself. Many proteins are too small to be studied even under a microscope, and must be crystallized in order to determine their 3-D structures. These structures tell researchers how a single protein functions and its involvement in the development of disease. Once modeled, drug developers can use the structure to develop a specific drug to interact with the protein, a process called structure-based drug design.
Two investigations, The Effect of Macromolecular Transport on Microgravity Protein Crystallization (LMM Biophysics 1) and Growth Rate Dispersion as a Predictive Indicator for Biological Crystal Samples Where Quality Can be Improved with Microgravity Growth (LMM Biophysics 3), will study the formation of these crystals, looking at why microgravity-grown crystals often are of higher quality than Earth-grown, and which crystals may benefit from being grown in space.
Rate of Growth – LMM Biophysics 1
Researchers know that crystals grown in space often contain fewer imperfections than those grown on Earth, but the reasoning behind that phenomenon isn’t crystal clear. A widely accepted theory in the crystallography community is that the crystals are of higher quality because they grow slower in microgravity due to a lack of buoyancy-induced convection. The only way these protein molecules move in microgravity is by random diffusion, a process that is much slower than movement on Earth.
Another less-explored theory is that a higher level of purification can be achieved in microgravity. A pure crystal may contain thousands of copies of a single protein. Once crystals are returned to Earth and exposed to an X-Ray beam, the X-ray diffraction pattern can be used to mathematically map a protein’s structure.
“When you purify proteins to grow crystals, the protein molecules tend to stick to each other in a random fashion,” said Lawrence DeLucas, LMM Biophysics 1 primary investigator. “These protein aggregates can then incorporate into the growing crystals causing defects, disturbing the protein alignment, which then reduces the crystal’s X-ray diffraction quality.”
The theory states that in microgravity, a dimer, or two proteins stuck together, will move much slower than a monomer, or a single protein, giving aggregates less opportunity to incorporate into the crystal.
“You’re selecting out for predominantly monomer growth, and minimizing the amount of aggregates that are incorporated into the crystal because they move so much more slowly,” said DeLucas.
The LMM Biophysics 1 investigation will put these two theories to the test, to try to understand the reason(s) microgravity-grown crystals are often of superior quality and size compared to their Earth-grown counterparts. Improved X-ray diffraction data results in a more precise protein structure and thereby enhancing our understanding of the protein’s biological function and future drug discovery.
Crystal Types – LMM Biophysics 3
As LMM Biophysics 1 studies why space-grown crystals are of higher quality than Earth-grown crystals, LMM Biophysics 3 will take a look at which crystals may benefit from crystallization in space. Research has found that only some proteins crystallized in space benefit from microgravity growth. The shape and surface of the protein that makes up a crystal defines its potential for success in microgravity.
“Some proteins are like building blocks,” said Edward Snell, LMM Biophysics 3 primary investigator. “It’s very easy to stack them. Those are the ones that won’t benefit from microgravity. Others are like jelly beans. When you try and build a nice array of them on the ground, they want to roll away and not be ordered. Those are the ones that benefit from microgravity. What we’re trying to do is distinguish the blocks from the jelly beans.”
Understanding how different proteins crystallize in microgravity will give researchers a deeper view into how these proteins function, and help to determine which crystals should be transported to the space station for growth.
“We’re maximizing the use of a scarce resource, and making sure that every crystal we put up there benefits the scientists on the ground,” said Snell.
Filed Under: Drug Discovery



