 Researchers have shown how controlling cholesterol metabolism in pancreatic cancer cells reduces metastasis, pointing to a potential new treatment using drugs previously developed for atherosclerosis.
Researchers have shown how controlling cholesterol metabolism in pancreatic cancer cells reduces metastasis, pointing to a potential new treatment using drugs previously developed for atherosclerosis.
“We show for the first time that if you control the cholesterol metabolism you could reduce pancreatic cancer spread to other organs,” said Ji-Xin Cheng, a professor in Purdue University’s Weldon School of Biomedical Engineering and Department of Chemistry. “We chose pancreatic cancer to test this approach because it is the most aggressive disease of all the cancers.”
Cheng had previously led a team of researchers discovering a link between prostate cancer’s aggressiveness and the accumulation of a compound produced when cholesterol is metabolized in cells, findings that could bring new diagnostic and treatment methods. The new study involved researchers at the Purdue Center for Cancer Research and School of Biomedical Engineering, the Indiana University Simon Cancer Center and School of Medicine, Purdue’s Department of Biological Sciences, Department of Comparative Pathobiology, and Department of Biochemistry.
The findings, detailed in a paper published on May 2 in the journal Oncogene, suggest a class of drugs previously developed to treat atherosclerosis could be repurposed for treatment of pancreatic cancer and other forms of cancer. Atherosclerosis is the buildup of fats, cholesterol and other substances in arteries, restricting blood flow.
The researchers found accumulations of the compound cholesteryl ester in human pancreatic cancer specimens and cell lines, demonstrating a link between cholesterol esterification and metastasis. Esterification is a biochemical process that allows cholesterol to be stored in cells. Excess quantities of cholesterol result in cholesteryl ester being stored in lipid droplets within cancer cells.
“The results of this study demonstrate a new strategy for treating metastatic pancreatic cancer by inhibiting cholesterol esterification,” said Jingwu Xie, the Jonathan and Jennifer Simmons Professor at the Indiana University School of Medicine and a researcher at the Indiana University Melvin and Bren Simon Cancer Center.
The paper’s lead author is Purdue post-doctoral fellow Junjie Li. Purdue researchers have developed an analytical tool called Raman spectromicroscopy that allows compositional analysis of single lipid droplets in living cells.
“We identified an aberrant accumulation of cholesteryl ester in human pancreatic cancer specimens and cell lines,” Li said. “Depletion of cholesterol esterification significantly reduced pancreatic tumor growth and metastasis in mice.”
Findings show that drugs like avasimibe, previously developed for treatment of atherosclerosis, reduced the accumulation of cholesteryl ester. The disease usually kills within a few months of diagnosis. It is hoped the potential new treatment might extend life of pancreatic cancer patients for a year, Cheng said.
The accumulation of cholesteryl ester is controlled by an enzyme called ACAT-1, and findings correlated a higher expression of the enzyme with a poor survival rate for patients. The researchers analyzed tissue samples from pancreatic cancer patients and then tested the drug treatment in a type of laboratory mice referred to as an orthotopic mouse model, developed at the IU School of Medicine. Specimens of human pancreatic tissues were obtained from the Simon Cancer Center Solid Tissue Bank.
Imaging showed a decrease of the number of lipid droplets, and Raman spectral analysis verified a significant reduction of cholesteryl ester in the lipid droplets, suggesting that avasimibe acted by blocking cholesterol esterification. The drug did not induce weight loss, and there was no apparent organ toxicity in the liver, kidney, lung and spleen, Cheng said.
Findings also showed that blocking storage of cholesteryl ester causes cancer cells to die, specifically due to damage to the endoplasmic reticulum, a workhorse of protein and lipid synthesis.
“By using avasimibe, a potent inhibitor of ACAT-1, we found that pancreatic cancer cells were much more sensitive to ACAT-1 inhibition than normal cells,” he said.
Additional research confirmed that the anti-cancer effect of avasimibe is specific to ACAT-1 inhibition. The researchers performed various biochemical assays and “genetic ablation” to confirm the drug’s anti-cancer effect.
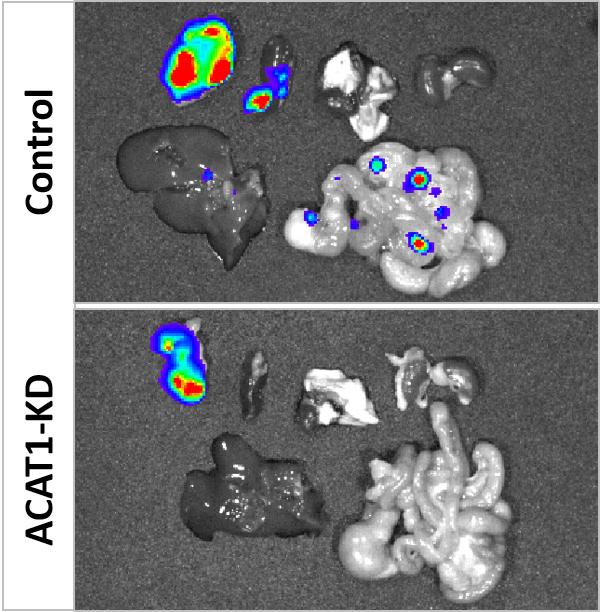
“The results showed that avasimibe treatment for four weeks remarkably suppressed tumor size and largely reduced tumor growth rate,” said paper coauthor Timothy Ratliff, the Robert Wallace Miller Director of Purdue’s Center for Cancer Research. “Metastatic lesions in lymph nodes and distant organs also were assessed at the end of the study. A much higher number of metastatic lesions in lymph nodes were detected in the control group than the avasimibe-treated group.”
Each mouse in the control group showed at least one metastatic lesion in the liver. In contrast, only three mice in the avasimibe treated group showed single lesion in liver.
Cheng, Li, and Ratliff have founded Resarci Therapeutics LLC at the Purdue Research Park to work toward developing a formulation of the drug for human cancer patients.
“We want to bring this to clinical use,” Cheng said.
The researchers will work with IU’s Xie to further study the potential treatment.
Filed Under: Drug Discovery



