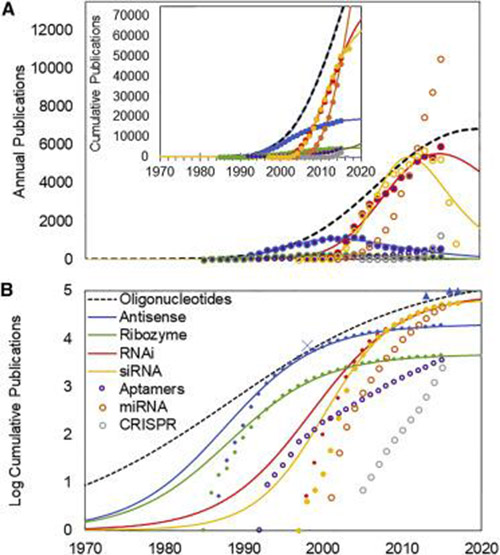
(A) Annual publications in PubMed related to oligonucleotide therapeutics (markers) and best fit TIME model (lines). The TIME model uses the best fit exponentiated logistic function based on the cumulative number of publications (inset graph). Data indicated with open markers could not be fit to this function. (B) The logistic (“S curve”) representation of the TIME model is shown on a log scale. Note that the apparent residuals are exaggerated at the low end of the log scale (see Materials and Methods). Dates of FDA approval of oligonucleotide therapeutics are indicated, including those for fomivirsen (1998, subsequently withdrawn from market), mipomersen (2013), nusinersen (2016), and eteplirsen (2016). Photo: Center for Integration of Science and Industry, Bentley University
The recent approval of Spinraza (nusinersen), jointly developed by Ionis Pharmaceuticals and Biogen, marks the arrival of a new class of biological products – oligonucleotide therapeutics. A recent publication from the Center for Integration of Science and Industry at Bentley University shows that the thirty year path from the initiation of research on oligonucleotides as therapeutics to the emergence of effective products followed predictable patterns of innovation, in which novel products are successfully developed only after the underlying basic research reaches a requisite level of maturity.
The article in the journal Molecular Therapy – Nucleic Acids titled “As Technologies for Nucleotide Therapeutics Mature, Products Emerge” describes the analysis of more than 60,000 research publications related to oligonucleotide therapies. The work applies a data analysis model for the growth and maturation of biomedical research to characterize the progress of research on the pharmaceutical chemistry of oligonucleotide therapeutics, as well as the molecular targets, for their actions. Previous work has demonstrated that successful product development characteristically follows the maturation of the enabling research past an analytically defined established point. The present work shows that the approval of oligonucleotide therapeutics exhibits a similar pattern.
Oligonucleotide therapeutics are a novel class of biopharmaceutical products comprised of short strings of synthetic nucleotides, which resemble the building blocks for DNA. These products are designed to alter the expression of target genes in the body, suppressing expression of genes associated with disease, or restoring expression of genes required for health. Originally described in the 1980s, the potential of oligonucleotides as therapeutic agents spawned hundreds of clinical trials and billions of dollars of investment in companies developing these therapeutics without generating successful products.
“Discovery and development of new medicines, such as an oligonucleotides, requires extensive basic research to optimize the product chemistry, improve its biological activity, and characterize a specific chemical entity that can safely treat diseases,” said Dr. Fred Ledley, director of the Center for Integration of Science and Industry. “Our work suggests that when data analytics are used to track the maturation of basic research, drug developers will be able to more efficiently recognize the point at which the science is mature enough for product development.”
Filed Under: Genomics/Proteomics




