FDA has announced that it has signed off on the use of COVID-19 therapy Veklury (remdesivir) from Gilead Sciences (Nasdaq:GILD) to include children at least 28 days old who weigh at least 3 kg (roughly 7 lb) and test positive results for COVID-19. The indication is limited to children who are either hospitalized or have…
FDA extends approval of remdesivir to encompass non-hospitalized high-risk
Gilead Sciences’ (NSDQ:GILD) Veklury (remdesivir) became the first FDA-approved COVID-19 drug when it was approved in October 2020. Now, the company has announced that the FDA has granted expedited approval of a supplemental new drug application (sNDA) for the drug to treat non-hospitalized adult and adolescent patients with a high risk of progression to severe…
COVID drugs come in 3 flavors; it’s time for more diversity
Our industry’s response to COVID-19 defied the conventional wisdom that it takes years to deliver new drugs. Makers of monoclonal antibodies led the pack, most notably Regeneron (NSDQ:REGN), which manufactured the 8-g, two-antibody cocktail administered to then-President Donald Trump last fall. Eli Lilly (NYSE:LLY) and Vir Biotechnology (NSDQ:VIR) pulled off similar feats. Prior speed records…
WHO to test three anti-inflammatory therapies in COVID-19 patients
The World Health Organization (WHO) is adding three anti-inflammatory therapies to its global Solidarity COVID-19 trial. WHO is referring to the expanded trial as “Solidarity PLUS.” WHO will investigate the following drugs as potential treatments for hospitalized COVID-19 patients: Ipca Laboratories’ artesunate is an FDA-indicated treatment for severe malaria in adult and pediatric patients. Last year,…
34 of the most innovative pharmaceutical products
The Galien Foundation has revealed its latest nominees for the 2021 Prix Galien USA Award highlighting innovations in biotechnology, pharmaceutical agents, medical technology and digital health products. Entrants to the competition must have received FDA approval within the past five years and demonstrate exceptional therapeutic potential. The Galien Foundation does not use financial data to…
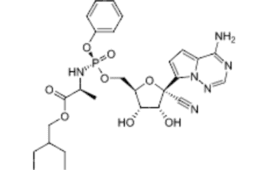
Remdesivir — a new blockbuster
In one sense, Veklury (remdesivir) is not a new drug. Gilead Sciences initially developed remdesivir (GS-5734) in reaction to the 2014 Ebola outbreak. The drug remained investigational, however, until the COVID-19 pandemic. In 2020, it became the first FDA-approved drug to treat COVID-19. Last year, it raked in $2.811 billion in revenue.
Roche retools COVID-19 strategy
The Swiss pharma giant Roche (OTCMKTS:RHHBY) has canceled two Phase 2 COVID-19 studies while looking to identify a new site to conduct a clinical study for the oral antiviral AT-527, which it is developing with Atea Pharmaceuticals (NSDQ:AVIR.O). The two companies were looking to launch a trial in the U.K., but falling COVID-19 cases there have…
Study: Remdesivir supports clinical improvement in hospitalized COVID-19 patients
A multicenter study involving a substantial number of minorities indicated that remdesivir supported clinical improvement in the majority of patients. The hospitalized remdesivir group had an average time to clinical improvement of five days versus seven days for those not receiving the drug. Remdesivir recipients had a 28-day mortality rate of 7.7% compared with a…
3 notable types of innovative drugs from 2020
Last year, FDA approved 53 drugs, leading the industry to describe 2020 as “a strong year for new drug therapy.” There are several drugs that stand out, according to Todd Wills, the co-author of a study that analyzes how innovative drugs are based on their structure. The drugs that follow are examples of notable innovative therapies.…
Questions dog Gilead’s COVID-19 drug remdesivir after FDA approval
Biotech firm Gilead Sciences is on the defensive as its revenue surges thanks to the COVID-19 drug Veklury, better known as remdesivir. Several press outlets are skeptical of the promise of remdesivir, the first COVID-19 treatment to win FDA approval. This week, Healthline questioned whether FDA should have approved the drug. The New York Times…