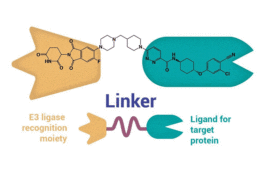
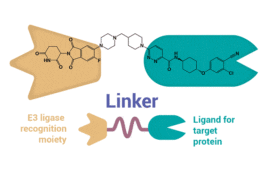
PROteolysis TArgeting Chimera (PROTAC)* drugs emerged in 2001 as a novel therapeutic option for treating previously untreatable diseases. To date, no PROTAC drugs have earned U.S. FDA approval, but the technology has opened up a promising new chapter in drug discovery. PROTACs are being widely explored across both industry and academia and have expanded from…
Tackling the DMPK challenges of developing PROTAC drugs
PROTACs, or PROteolysis TArgeting Chimera, emerged as novel therapeutic modalities in 2001 but have only begun emerging from clinical trials (PROTAC® is a registered trademark of Arvinas. In this article, PROTAC specifically refers to the abbreviation of PROteolysis TArgeting Chimera as therapeutic modalities). PROTACs’ unique structures and modes of action (MOA) differentiate them from conventional…
Insilico Medicine announces PROTAC partnership with Arvinas
Insilico Medicine (Hong Kong) has entered into an R&D pact with Arvinas (NSDQ:ARVN) to explore applications of Insilico’s AI technology to PROTACs. By regulating protein function, PROTACS can optimize the sensitivity to drug-resistant targets, as an article in Signal Transduction and Targeted Therapy notes Insilico and Arvinas will partner in designing treatment modalities for current and next-generation…