A recent review of more than 2,000 studies related at least indirectly to obesity on clinicaltrials.gov highlights the pronounced significant shift in research focus toward GLP-1 receptor agonists for a range of indications with at least some involvement of metabolic disorders. The site, which provides a robust but not-exhaustive snapshot of clinical trial activity, cites…
Best-selling pharmaceuticals of 2023 reveal a shift in pharma landscape
Note: This feature on the best-selling pharmaceuticals of 2023 was updated on March 27. Changes are possible as more data become available. Sales of drugs with more than one developer are added together (as in the case of the COVID-19 vaccine Comirnaty) unless one of those companies records the net sales for it, i.e., Dupixent.…
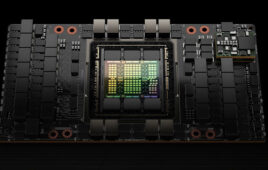
Denmark teams up with Novo Nordisk Foundation, NVIDIA to launch visionary AI research center
A collaboration between the Novo Nordisk Foundation, the Export and Investment Fund of Denmark (EIFO), and NVIDIA will establish a national AI Innovation Centre in Denmark focused on accelerating research and innovation in fields including healthcare, life science, and quantum computing. The initiative is led on the Danish side by the Novo Nordisk Foundation, which…
How Novo Nordisk’s Wegovy cardiovascular benefits could drive further growth
Semaglutide was already one of the best-selling drugs of recent memory. And Novo Nordisk the fastest-growing Big Pharma firm. Now, the FDA’s decision to expand the label of its weight-loss version of the drug to include cardiovascular benefits could help unlock more growth momentum for Novo Nordisk. This positions Wegovy as the first weight-loss medication…
Novo Nordisk stops once-weekly semaglutide kidney outcomes trial early following interim analysis
Novo Nordisk will halt the phase 3b FLOW trial, which investigated the effects of once-weekly injectable semaglutide on kidney outcomes in individuals with type 2 diabetes (T2D) and chronic kidney disease (CKD). The company reached the decision following the recommendation of the independent Data Monitoring Committee (DMC), following an interim analysis that met pre-specified criteria…
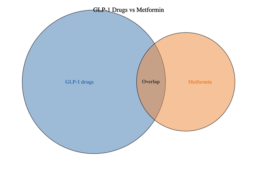
An overview of the GLP-1 landscape in obesity therapeutics
The GLP-1 drug market continues to boom lately thanks to highly effective new medications like Novo Nordisk’s Wegovy and continued demand for the drug class in diabetes. The runaway success of Wegovy and Ozempic (both formulations of semaglutide) has caused Novo Nordisk’s share price to skyrocket in 2023, briefly making the Danish pharmaceutical company the most…
The weight-loss drug market: overhyped or justified?
The obesity drug market has seen a surge of interest recently, largely thanks to the popularity of glucagon-like peptide-1 (GLP-1) drugs like Novo Nordisk’s Wegovy and Eli Lilly’s Mounjaro. The explosion in interest in the drug class has fueled the stock prices of Novo and Lilly, which both have multiple GLP-1 drugs in their portfolios. …
Roche shares upbeat Phase 3 data for Hemlibra in hemophilia
Facing potential competition from Novo Nordisk’s (NYSE:NVO) investigational blood coagulation factor stimulants Mim8, Roche (SIX:RO, ROG; OTCQX:RHHBY) released positive new data from the Phase 3 HAVEN 6 study focused on the use of Hemlibra (emicizumab) in patients with mild or moderate hemophilia A. Hemlibra won FDA approval in 2018 for hemophilia A without factor VIII…
Semaglutide supply shortfall fuels demand for alternatives
Novo Nordisk (NYSE:NVO) continues to face supply shortages for its Wegovy (semaglutide) pens, which are FDA approved for weight management. Against that backdrop, a growing number of companies have begun selling compounded versions of the drug. Drug compounding involves “combining, mixing, or altering ingredients to create a medication tailored to the needs of an individual patient,”…
10 leading pharma executives you need to know
Given the complexity of the pharmaceutical business and the generally slow pace of new drug development, it can be challenging to identify leading pharma executives. Compensation is not necessarily an accurate barometer of success. That said, CEOs’ annualized return over tenure as CEO over their term can hint at performance. In addition, rating sites like Glassdoor…
15 of the best pharma companies to work for
The pharmaceutical industry continues to see strong growth and is poised to have a compound annual growth rate of 13.7% from 2020 to 2027, according to projections from Grand View Research. The best pharma companies to work for continue to offer a number of advantages to skilled workers. Pharma positions remain in high demand, and…
Novo Nordisk wins FDA approval for higher-dose Ozempic for adults with type 2 diabetes
FDA has approved a 2 mg injectable dose of Novo Nordisk’s (NYSE:NVO) Ozempic (semaglutide), a once-weekly glucagon-like peptide-1 (GLP-1) analog. The indication covers improving blood sugar control and reducing the risk of cardiovascular complications in adults with type 2 diabetes when used in conjunction with diet and exercise changes. To win the new indication, the…
J&J and Roche named to Clarivate’s Top 100 Global Innovators list
Few healthcare companies were included in the annual ranking of innovative companies from the analytics firm Clarivate plc (NYSE:CLVT). Two companies in pharma and another in medtech, however, made the cut. Johnson & Johnson was featured for the second consecutive year as a top 100 Global innovator. Roche was included for the 11th consecutive year…
Noninvasive liver exam plays role in fighting viral hepatitis and fatty liver disease
Millions of Americans who are living with hepatitis C virus (HCV) will develop a chronic infection that, if left untreated, can cause serious health problems, including liver disease, cirrhosis, liver failure and liver cancer. More than one-third of HCV-infected individuals progress to advanced fibrosis and cirrhosis, and among those with cirrhosis, about 3–5% per year…
Lilly’s tirzepatide bests semaglutide in type 2 diabetes trial
Eli Lilly (NYSE:LLY) has announced that its investigational drug tirzepatide led to more substantial blood glucose and body weight improvements in a Phase 3 trial than semaglutide, a diabetes drug from Novo Nordisk (NYSE:NVO) that recently scored FDA approval for weight loss in early June. Lilly’s SURPASS-2 results published in The New England Journal of Medicine show tirzepatide…
FDA approves Novo Nordisk’s semaglutide for weight management
FDA has approved Wegovy, the weekly semaglutide treatment for obesity from Novo Nordisk (CPH:NOVO-B). The agency approved the use of semaglutide for type 2 diabetes in 2017. FDA approved an oral formulation for diabetes in 2019. Semaglutide is a glucagon-like peptide (GLP-1) receptor agonist (RA) that continues to find wider use. In clinical trials, Novo Nordisk people…
Why Lilly’s tirzepatide has blockbuster potential
Tirzepatide from Eli Lilly (NYSE:LLY) continues to show promise for diabetes, outperforming popular diabetes drugs in head-to-head clinical trials, according to GlobalData. In the recent SURPASS-4 study, tirzepatide supported the reduction of hemoglobin A1C (HbA1c) in people with type 2 diabetes while also supporting weight loss. The study pitted tirzepatide against insulin glargine. Tirzepatide is a dual glucose-dependent insulinotropic…
Novo Nordisk to test oral semaglutide as an obesity therapy
Global pharma firm Novo Nordisk (NYSE:NVO) will launch a Phase 3a study to investigate the potential of oral Ozempic (semaglutide) to treat obesity. The drug is currently indicated in a subcutaneous form in the U.S. for people with type 2 diabetes to improve glycemic control in conjunction with diet and exercise and to lower diabetics’…
Semaglutide shows promise for weight loss maintenance
Signs are growing that the diabetes drug Ozempic (semaglutide) from Novo Nordisk can support weight loss in overweight and obese individuals. A recent JAMA study involving overweight and obese individuals gave recipients a 2.4-mg subcutaneous dose of semaglutide once weekly for 20 weeks. After that period, investigators continued administering the drug to half of the participants while…
FDA sends Novo Nordisk refusal to file letter for weekly 2-mg semaglutide
Novo Nordisk announced that the FDA issued a refusal-to-file letter related to its application to expand the label for once-weekly subcutaneous Ozempic (semaglutide). The company had sought to expand the label for a 2.0-mg dose of once-weekly semaglutide to treat type 2 diabetes. It filed the letter on Jan. 20, 2021. The letter indicates that…
Semaglutide paired with behavioral therapy tripled weight loss in trial
The diabetes drug Ozempic (semaglutide) could potentially enhance weight loss in overweight and obese patients without diabetes, according to a study recently published in JAMA and The New England Journal of Medicine. In December, Novo Nordisk (NYSE:NVO) submitted a new drug application to the FDA for a 2.4-mg dose of subcutaneous semaglutide for chronic weight management. The drug won FDA…
Dicerna and Novo Nordisk to develop RNAi therapies for liver-related cardio-metabolic diseases
Dicerna Pharmaceuticals (NSDQ:DRNA) announced today that it closed a discovery & development agreement with Novo Nordisk (NYSE:NVO) worth at least $175 million to further development of therapies for treating liver-related cardio-metabolic diseases. The two companies announced the agreement on Nov. 18, 2019, outlining the plan to discover and develop the therapies using Dicerna’s GalXC RNAi platform…