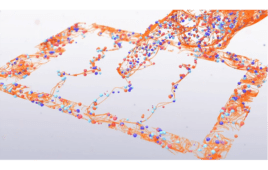
Pharmaceutical and biotechnology organizations have made significant progress enabling accelerated drug discovery with traditional laboratory and computing methods. However, they have only just begun to harness the potential of artificial intelligence (AI) to develop more effective therapeutics faster. This article looks at recent work from researchers at GlaxoSmithKline (GSK) with Cerebras Systems as an example…
GSK halts Phase 3 RSV maternal vaccine candidate program
GlaxoSmithKline plc (LSE/NYSE:GSK) has announced that it has chosen to stop enrollment and vaccination of studies testing its respiratory syncytial virus (RSV) maternal vaccine candidate in women. The company had announced a voluntary pause of the trials NCT04605159, NCT04980391 and NCT05229068 on February 18. GSK did not elaborate on the reason for halting the trials.…
Medicago and GSK win approval from Health Canada for adjuvanted plant-based COVID-19 vaccine
GlaxoSmith Kline (NYSE:GSK) and Sanofi (NYSE:SNY) recently announced their intent to seek regulatory authorization for an adjuvanted recombinant protein-based COVID-19 vaccine. Now, GSK and its partner Medicago have received approval from Health Canada for another COVID-19 vaccine, which combines the former’s adjuvant technology with the latter’s plant-derived vaccine. Known as Covifenz, the vaccine makes use of plant-based virus-like…
Sanofi and GSK aim to commercialize COVID-19 vaccine
In the U.S., Moderna (NASDAQ:MRNA) and Pfizer (NYSE:PFE) continue to dominate the COVID-19 landscape while demand for Johnson & Johnson’s (NYSE:JNJ) vaccine remains limited. Now, Sanofi (NASDAQ:SNY) and GSK (NYSE:GSK) are preparing to get into the game by preparing their regulatory submissions for their COVID-19 vaccine. The companies are currently communicating with the FDA and…
Chinese regulators approve GSK’s Benlysta for lupus nephritis
GlaxoSmithKline plc (LSE/NYSE:GSK) has announced that China’s National Medical Products Administration (NMPA) has approved Benlysta (belimumab) for the treatment of adult patients with active lupus nephritis (LN), a type of kidney disease affecting more than half of those with systemic lupus erythematosus (SLE). Benlysta is the only biologic approved to treat SLE and lupus nephritis…
CureVac and GSK launch Phase 1 trial of mRNA-based flu vaccine
CureVac (NSDQ:CVAC) and GSK (NYSE:GSK) have announced the dosing of the first participant in a Phase 1 study of a multivalent influenza vaccine built on a second-generation mRNA backbone. The study is based in Panama and will enroll approximately 240 subjects. CVAC shares increased 2.27% to $19.34 in mid-day trading. At various points last year,…
Biotech Altos Labs emerges with $3B in funding to focus on ‘cellular rejuvenation programming’
The biotech Altos Labs has exited stealth mode with top-tier talent and $3 billion in funding with a broad mission of promoting homeostasis in cells to reverse disease, injury and disability. In other words, the company aims to use biotechnology to fight aging and disease, as early media reports have suggested. In a press release, Altos…
How Vir Biotechnology intends to functionally cure HIV and prevent malaria
Vir Biotechnology (NSDQ:VIR) with its partner GSK (LSE/NYSE:GSK) recently announced growing traction for the investigational COVID-19 monoclonal antibody sotrovimab. Now, San Francisco–based Vir has expanded its partnership with the Bill & Melinda Gates Foundation to focus on developing broadly neutralizing antibodies intended to provide a “vaccinal effect” for treating HIV and preventing malaria. Vir Biotechnology…
GSK and Vir file for EUA for intramuscular administration of sotrovimab to treat COVID-19
GlaxoSmithKline (LSE/NYSE GSK) and Vir Biotechnology (NSDQ:VIR) are seeking to expand the use of the investigational monoclonal antibody sotrovimab. Intravenous sotrovimab is currently authorized to treat mild-to-moderate COVID-19 in patients at least 12 years of age, weighing at least 40 kg (88 pounds). The authorization is constrained to patients who face a high risk of…
7 potential applications of mRNA-based therapies
Scientists have experimented with mRNA for decades, but the pandemic foisted the platform into the limelight. The Pfizer-BioNTech (NYSE:PFE/NSDQ:BNTX) and Moderna (NSDQ:MRNA) COVID-19 vaccines have since emerged as two of the best-selling pharmaceutical products in recent memory. Researchers are now exploring dozens of new possibilities for the mRNA platform. Here, we summarize several areas where…
Shingles vaccination may lower risk of severe COVID-19, study finds
A large retrospective study found that a recombinant adjuvanted zoster vaccine was associated with a 32% reduced risk of COVID-19-associated hospitalization. The study, published in The Journal of Infectious Diseases, also found that individuals who received at least one dose of the Shingrix vaccine were 16% less likely to be diagnosed with COVID-19 during the trial…
Sotrovimab wins EU marketing authorization as an early COVID-19 therapy
GlaxoSmithKline (LSE/NYSE:GSK) and its partner Vir Biotechnology (NSDQ:VIR) have received marketing authorization from the European Commission for the dual-action monoclonal antibody Xevudy (sotrovimab). The marketing authorization covers individuals 12 and older who weigh at least 40 kg who do not need supplemental oxygen. It also requires that such individuals have a heightened risk of developing…
Ivy Brain Tumor Center partners with GSK and UCSF to test Zejula in brain cancer patients
The Ivy Brain Tumor Center (Phoenix) and University of California, San Francisco will partner on a Phase 0 clinical trial to test GSK’s (NYSE:GSK) Zejula in patients with newly diagnosed glioblastoma and recurrent glioma (grades II–IV). Zejula (niraparib) is a daily oral poly (ADP-ribose) polymerase (PARP) inhibitor. FDA introduced Phase 0 clinical trials in 2004…
Sanofi is banking on a $10 COVID-19 vaccine
Sanofi (NSE:SANOFI) and its partner GSK (NYSE:GSK) are hoping the low price of their experimental COVID-19 vaccine will stoke interest. In a call with analysts, Thomas Triomphe, head of the Sanofi Pasteur vaccines division, said that its vaccine would cost less than $10 per dose. For the sake of comparison, Pfizer-BioNTech and Moderna COVID-19 vaccines generally cost…
Bayer abandons plans to produce first-generation CureVac vaccine
Bayer (ETR:BAYN) had planned to retool a facility in Wuppertal, Germany, to produce CureVac’s (NSDQ:CVAC) COVID-19 vaccine. But Leverkusen, Germany–headquartered Bayer has put those plans on ice, according to the German paper Leverkusener Anzeiger. Bayer had planned on producing 160 million doses of the vaccine annually. In June, CureVac reported that its COVID-19 vaccine was 48% efficacious…
Janssen begins Phase 3 study for RSV vaccine in adults 60 and older
After recently sharing positive Phase 2b data related to its experimental respiratory syncytial virus (RSV) vaccine in seniors, Janssen (NYSE: JNJ) has launched a Phase 3 study of the vaccine. RSV is common and contributes to serious respiratory illnesses, including pneumonia in the elderly. The virus infects approximately 64 million people annually. The global EVERGREEN…
Caribou Biosciences adds Nancy Whiting to its board of directors
CRISPR genome-editing biopharma firm Caribou Biosciences (NSDQ:CRBU) has appointed Nancy Whiting to its board of directors. Whiting had previously worked at Seagen (formerly known as Seattle Genetics) for 14 years and had three years of experience at GSK. At Seagen, she was involved in the development of several drugs, including Adcetris, Padcev, Tukysa, tisotumab vedotin…
NVIDIA debuts supercomputer for healthcare and AI research
Santa Clara, Calif.–based NVIDIA has launched the Cambridge-1, which it hails as “the United Kingdom’s most powerful supercomputer.” Developed with healthcare applications in mind, NVIDIA invested some $100 million in the supercomputer. Initial applications of the Cambridge-1 include using AI to improve drug development, explore the causes of dementia and identify genomic-based disease risk factors.…
An inside look at GSK’s digital twin initiative
Digital twins, functional computerized models of physical objects, are a staple of smart manufacturing. Their use in the pharmaceutical industry, however, is still in an early phase. GlaxoSmithKline (LON:GSK) is one of the first pharmaceutical companies to announce a digital twin initiative. Partnering with Siemens (ETR:SIE) and Atos (EPA:ATO), GSK has created a real-time simulation of…
CureVac’s interim vaccine efficacy is 47% in Phase 2b/3 study
Germany-based CureVac (NSDQ:CVAC) announced that its mRNA vaccine was 47% effective against COVID-19 in a second interim analysis of a pivotal study involving approximately 40,000 participants in 10 countries. The rise of COVID-19 variants played a role in the disappointing results. At least 13 COVID-19 variants were present in the study population who contracted the…
How lupus clinical trials are evolving
In the past half-century, scores of investigational drugs for lupus have seemingly failed in clinical trials. GSK’s Benlysta (belimumab) is unique in winning approval from the FDA and European regulatory authorities. Anifrolumab from AstraZeneca, which would be a first-in-class type I interferon inhibitor, is one of the most promising investigational drugs for treating systemic lupus…
CureVac’s COVID-19 vaccine candidate inches forward to Phase 2b/3 efficacy readout
CureVac (NSDQ:CVAC) has announced that its first-generation COVID-19 vaccine has passed its first interim analysis but has chosen not to share efficacy data until a statistically significant efficacy analysis is ready. The Tübingen, Germany–headquartered company is developing two mRNA-based vaccines. The mRNA vaccines from Moderna (NSDQ:MRNA) and Pfizer (NYSE:PFE) are currently two of the most popular…
Sanofi and GSK start Phase 3 trial of COVID-19 vaccine
Sanofi and GlaxoSmithKline announced today that they began enrollment in the Phase 3 clinical trial for their COVID-19 vaccine candidate. The companies will evaluate the safety, efficacy and immunogenicity of their adjuvanted recombinant-protein COVID-19 vaccine candidate in a global, randomized, double-blind, placebo-controlled Phase 3 study of more than 35,000 participants aged 18 and older, according…
CureVac and GSK announce promising preclinical data for next-gen COVID-19 vaccine
As CureVac (NSDQ:CVAC) awaits European authorization for its mRNA COVID-19 vaccine, it is conducting preclinical research on a second-generation vaccine candidate known as CV2CoV, which it is developing with GSK. In a study involving rats, the new mRNA vaccine candidate yielded high levels of antigen and strong neutralizing antibody titers after the first vaccination. Tübingen, Germany–based…
GSK and Vir Biotechnology seek EUA for COVID-19 monoclonal antibody
GlaxoSmithKline and Vir Biotechnology have filed for emergency use authorization (EUA) for VIR-7831 (GSK4182136), a dual-action SARS-CoV-2 monoclonal antibody. VIR-7831 is designed to stop SARS-CoV-2 viral entry into cells and accelerate the clearing of infected cells. VIR-7831 would have a similar indication to other monoclonal antibodies that have won an EUA. It is intended for…