Ozempic, Rybelsus and Wegovy have transformed the diabetes and weight loss treatment landscape, but when it comes to the impact of their active ingredient, semaglutide, on fetal development, “the answer is we do not know,” said Dr. Marijane Hynes, clinical professor of medicine at the George Washington University School of Medicine and Health Sciences. Hynes…
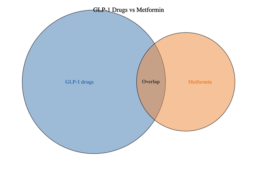
GLP-1s overtake metformin in metabolic clinical trials by a wide margin: A visual exploration
A recent review of more than 2,000 studies related at least indirectly to obesity on clinicaltrials.gov highlights the pronounced significant shift in research focus toward GLP-1 receptor agonists for a range of indications with at least some involvement of metabolic disorders. The site, which provides a robust but not-exhaustive snapshot of clinical trial activity, cites…
Could Wegovy’s cardiovascular label expansion be a catalyst for GLP-1 obesity drug coverage?
The recent FDA approval of a cardiovascular risk reduction indication for Wegovy (semaglutide) could point toward a significant opportunity for pharma companies seeking to reshape payer perceptions and expand coverage for next-gen metabolic therapies. This regulatory shift, allowing Wegovy to be prescribed for reducing the risk of major adverse cardiovascular events such as heart attack…
GLP-1s, ADCs, AI and the future of pharma
Pharma’s potential breakthroughs in AI, ADCs, and GLP-1 receptor agonists raise a critical question: can innovation outpace the relentless rise of chronic disease? The IQVIA Institute for Human Data Science sheds light on this theme, among many others, in its 80-page Global Trends in R&D 2024 report. Pillar 1: GLP-1 receptor agonists targeting metabolic disease…
Beyond diabetes and obesity: Can GLP-1 therapies also transform chronic disease treatment?
Glucagon-like peptide-1 (GLP-1) receptor agonists like semaglutide and tirzepatide have cemented their status as two of the most successful drugs in recent memory. Recent projections have estimated that the drug class could fetch $44 billion by 2030 and $71 billion by 2032. But GLP-1 sales could potentially reach greater heights as these therapies move beyond…
GLP-1 drugs could open a new frontier in NASH treatment
This morning, Eli Lilly reported positive phase 2 results for its dual GLP-1 and GIP receptor agonist tirzepatide in patients with nonalcoholic steatohepatitis (NASH). In the SYNERGY-NASH trial, the therapy achieved NASH resolution without worsening fibrosis in 61.3% of patients. That is considerably higher than data for semaglutide. Picturing tirzepatide’s NASH resolution in a phase…
Lilly’s Zepbound to enter the weight management market with competitive pricing
Lilly’s tirzepatide notched an FDA approval for chronic weight management, potentially clearly the way for billions in additional sales. Analysts have projected that the drug could fetch $26 billion in annual sales by 2030, with roughly two-thirds of that sum related to obesity treatment. Bank of America analyst Geoff Meacham is even more optimistic, predicting…
Will GLP-1 drugs transition from obesity and diabetes to diverse clinical indications?
The explosive sales growth of GLP-1 drugs has analysts projecting that the antiobesity drugs could be a $44 billion market by 2030. Some observers are more upbeat, projecting that the sector could be worth more than $100 billion in the coming years. Pfizer CEO Albert Bourla projects that the market will reach $90 billion by…
Tirzepatide versus semaglutide: Which contender will prevail in the battle against obesity and type 2 diabetes?
Eli Lilly‘s (NYSE:LLY) tirzepatide achieved up to 15.7% weight loss in the SURMOUNT-2 study, sparking a potential tirzepatide versus semaglutide competition in the obesity and type 2 diabetes treatment markets. The phase 3 study enrolled 938 participants with diverse backgrounds. Tirzepatide promises to be a megablockbuster with a number of analysts pegging peak annual sales…