Note: This feature on the best-selling pharmaceuticals of 2023 was updated on March 27. Changes are possible as more data become available. Sales of drugs with more than one developer are added together (as in the case of the COVID-19 vaccine Comirnaty) unless one of those companies records the net sales for it, i.e., Dupixent.…
The 50 best-selling pharmaceuticals of 2022: COVID-19 vaccines poised to take a step back
The COVID-19 pandemic has had a profound impact on the best-selling pharmaceuticals, leading to shifts in the list with Pfizer and BioNTech’s Comirnaty surpassing AbbVie’s Humira for the No. 1 spot in 2021. That momentum continued in 2022, with Pfizer and BioNTech jointly raking in $59.1 billion in revenue from the sales of the COVID-19…
The top 100 cell and gene therapy companies to watch in 2023
The cell and gene therapy sector is poised to deliver a wave of new therapies with the potential to cure rare and common diseases. As many as 13 new cell or gene therapies could be approved for use in the U.S., Europe, or both by the end of 2023. While manufacturing and regulatory challenges remain,…
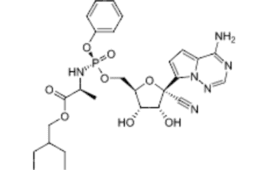
Gilead resubmits application to FDA for twice-yearly HIV drug lenacapavir
Gilead Sciences (Nasdaq: GILD) has asked FDA for the second time in a year to approve the capsid protein inhibitor lenacapavir. The drug won Breakthrough Therapy Designation from FDA in 2019 for HIV-1-infected individuals who are heavily treatment-experienced and have resistance to multiple drugs. The designation covered the potential use in tandem with other antiretroviral…
Gilead notches remdesivir indication to treat young children
FDA has announced that it has signed off on the use of COVID-19 therapy Veklury (remdesivir) from Gilead Sciences (Nasdaq:GILD) to include children at least 28 days old who weigh at least 3 kg (roughly 7 lb) and test positive results for COVID-19. The indication is limited to children who are either hospitalized or have…
J&J and Roche named to Clarivate’s Top 100 Global Innovators list
Few healthcare companies were included in the annual ranking of innovative companies from the analytics firm Clarivate plc (NYSE:CLVT). Two companies in pharma and another in medtech, however, made the cut. Johnson & Johnson was featured for the second consecutive year as a top 100 Global innovator. Roche was included for the 11th consecutive year…
Biktarvy shows high efficacy at five years in treatment-naïve adults with HIV
Gilead Sciences (NSDQ:GILD) announced that Biktarvy (bictegravir 50 mg/emtricitabine 200 mg/tenofovir alafenamide 25 mg tablets) offered sustained efficacy at week 240 in two Phase studies (Study 1489 and Study 1490). The Foster City, California–based company noted that there were no cases of treatment failure as a result of viral resistance detected in the studies. The…
Gilead to pay $1.25B in settlement with ViiV Healthcare
Gilead Sciences (NSDQ:GILD) will pay a 3% royalty on future U.S. sales of Biktarvy on top of a $1.25 billion upfront payment to settle global patent infringement litigation with ViiV Healthcare. The royalty payments will be in effect until the expiration of the applicable patent (No. 8,129,385) on 5 October 2027. Biktarvy generated $7.26 billion…
Kyverna Therapeutics wins $85 million in Series B financing
The cell therapy company Kyverna Therapeutics (Emeryville, California) has closed an oversubscribed $85 million Series B funding round led by Northpond Ventures. The company plans to use the funding to launch a Phase 2 trial for its lead asset, KYV-101, in the first half of 2022. KYV-101 is an autologous anti-CD19 chimeric antigen receptor T-cell…
FDA extends approval of remdesivir to encompass non-hospitalized high-risk
Gilead Sciences’ (NSDQ:GILD) Veklury (remdesivir) became the first FDA-approved COVID-19 drug when it was approved in October 2020. Now, the company has announced that the FDA has granted expedited approval of a supplemental new drug application (sNDA) for the drug to treat non-hospitalized adult and adolescent patients with a high risk of progression to severe…
Gilead Sciences, Merck near the top of Newsweek’s most responsible companies list
A handful of big names in drug discovery and development are among the 500 “most responsible,” according to Newsweek. The outlet published its “America’s Most Responsible Companies 2022” list, marking the third installment of the compilation (in partnership with Statista), this time expanded to include 500 of the largest public corporations around. Companies were judged with an overall…
Appeals court invalidates $1.2 billion fine against Gilead Sciences
The U.S. Court of Appeals for the Federal Circuit tossed a $1.2 billion fine against Gilead Sciences after invalidating portions of a Memorial Sloan Kettering Cancer Center patent licensed to a Bristol Myers Squibb subsidiary. The patent was the basis for the previous ruling against Foster City, Calif.–based Gilead, which related to patent infringement claims…
Novartis’s Kymriah fails in study focused on aggressive B-cell non-Hodgkin lymphoma
Novartis (SWX:NOVN) has announced that the genetically modified autologous T cell immunotherapy Kymriah (tisagenlecleucel) failed to meet the primary endpoint in its Phase 3 BELINDA trial. That endpoint involved event-free survival for people with aggressive B-cell non-Hodgkin lymphoma compared to standard of care. To qualify for the study, patients needed to have primary refractory disease…
Kite and Appia Bio announce on allogeneic cell therapy alliance
Gilead (NSDQ:GILD) subsidiary Kite will collaborate with Appia Bio (Culver City, Calif.) to develop engineered allogeneic cell therapies from hematopoietic stem cells (HSCs) for cancer patients. To that end, they have entered into a collaboration and license agreement to develop HSC-derived cell therapies for hematological malignancies. Under the agreement, Appia Bio will lead preclinical and…
34 of the most innovative pharmaceutical products
The Galien Foundation has revealed its latest nominees for the 2021 Prix Galien USA Award highlighting innovations in biotechnology, pharmaceutical agents, medical technology and digital health products. Entrants to the competition must have received FDA approval within the past five years and demonstrate exceptional therapeutic potential. The Galien Foundation does not use financial data to…
Remdesivir — a new blockbuster
In one sense, Veklury (remdesivir) is not a new drug. Gilead Sciences initially developed remdesivir (GS-5734) in reaction to the 2014 Ebola outbreak. The drug remained investigational, however, until the COVID-19 pandemic. In 2020, it became the first FDA-approved drug to treat COVID-19. Last year, it raked in $2.811 billion in revenue.
Pharma 50: Here’s how the world’s largest pharma companies are doing
The global pharmaceutical industry held up well during the pandemic, with 10 of the largest businesses only seeing a roughly –3% drop in revenue in 2020. Eight of the 10 even came out ahead. That’s one of the big takeaways from Drug Discovery & Development’s inaugural Pharma 50, a compilation of data on the largest…
Roche retools COVID-19 strategy
The Swiss pharma giant Roche (OTCMKTS:RHHBY) has canceled two Phase 2 COVID-19 studies while looking to identify a new site to conduct a clinical study for the oral antiviral AT-527, which it is developing with Atea Pharmaceuticals (NSDQ:AVIR.O). The two companies were looking to launch a trial in the U.K., but falling COVID-19 cases there have…
Study: Remdesivir supports clinical improvement in hospitalized COVID-19 patients
A multicenter study involving a substantial number of minorities indicated that remdesivir supported clinical improvement in the majority of patients. The hospitalized remdesivir group had an average time to clinical improvement of five days versus seven days for those not receiving the drug. Remdesivir recipients had a 28-day mortality rate of 7.7% compared with a…
Gilead’s lenacapavir shows promise for multi-drug-resistant HIV
Gilead Sciences presented new results from its Phase 2/3 CAPELLA study focused on its investigational, long-acting HIV-1 capsid inhibitor, lenacapavir. The trial, which involved heavily treatment-experienced people with multi-drug resistant HIV-1 infection, showed that lenacapavir had substantial virologic suppression for 26 weeks. In November, the company had announced that 88% of lenacapavir recipients in the…
3 notable types of innovative drugs from 2020
Last year, FDA approved 53 drugs, leading the industry to describe 2020 as “a strong year for new drug therapy.” There are several drugs that stand out, according to Todd Wills, the co-author of a study that analyzes how innovative drugs are based on their structure. The drugs that follow are examples of notable innovative therapies.…
How the clinical trial industry is making the most of COVID-19 disruption
Like most things in healthcare, clinical trials have been slow to evolve in recent decades. But the COVID-19 pandemic has forced clinical trial research teams to become more efficient, patient-centered and inclusive. Much is at stake as the pandemic has already caused significant clinical trial disruption. EY estimates that the COVID-19 impact on Phase 3…
Questions dog Gilead’s COVID-19 drug remdesivir after FDA approval
Biotech firm Gilead Sciences is on the defensive as its revenue surges thanks to the COVID-19 drug Veklury, better known as remdesivir. Several press outlets are skeptical of the promise of remdesivir, the first COVID-19 treatment to win FDA approval. This week, Healthline questioned whether FDA should have approved the drug. The New York Times…
Scholar Rock sees stock soar after announcing positive SMA results
A monoclonal antibody that blocks the activation of the skeletal muscle protein myostatin could be novel muscle-directed therapy for spinal muscular atrophy patients. Cambridge, Mass.–based Scholar Rock (NSDQ:SRRK), which is developing the SRK-015 investigational antibody, saw its shares more than double to $30.02 apiece yesterday. SRK-015 could lead to the formation of a new treatment…
FDA approves remdesivir as a COVID-19 treatment
The FDA announced that it fully approved the use of remdesivir as a treatment for COVID-19 requiring hospitalization in all adult and some pediatric patients. Remdesivir is only to be administered in a hospital or healthcare setting capable of providing acute care comparable to inpatient hospital care. The drug, also referred to by the FDA…