Pulmonary arterial hypertension (PAH), a rare, progressive and life-threatening blood vessel disorder, affects some 500 to 1,000 new patients each year in the U.S. FDA recently approved Opsynvi, a first-of-its-kind once-daily single-tablet combination therapy from Johnson & Johnson. “With this approval, our portfolio now includes treatments that address all three guideline-recommended pathways,” said a J&J…
Could Wegovy’s cardiovascular label expansion be a catalyst for GLP-1 obesity drug coverage?
The recent FDA approval of a cardiovascular risk reduction indication for Wegovy (semaglutide) could point toward a significant opportunity for pharma companies seeking to reshape payer perceptions and expand coverage for next-gen metabolic therapies. This regulatory shift, allowing Wegovy to be prescribed for reducing the risk of major adverse cardiovascular events such as heart attack…
How Novo Nordisk’s Wegovy cardiovascular benefits could drive further growth
Semaglutide was already one of the best-selling drugs of recent memory. And Novo Nordisk the fastest-growing Big Pharma firm. Now, the FDA’s decision to expand the label of its weight-loss version of the drug to include cardiovascular benefits could help unlock more growth momentum for Novo Nordisk. This positions Wegovy as the first weight-loss medication…
Could LSD change the game in anxiety treatment?
A once-controversial psychedelic substance could potentially be a promising treatment for generalized anxiety disorder (GAD). That’s the view of Dr. Rakesh Jain, a psychiatrist with extensive experience in clinical practice, research, and education, affiliated with Texas Tech University School of Medicine. Jain expressed optimism in LSD-based therapy while acknowledging the challenges inherent in such a…
A timeline of Aduhelm’s rise and fall
The story of Aduhelm has been rocky now for years. Biogen turned heads when its controversial Alzheimer’s therapy won accelerated approval from the FDA against the advice of its own advisory panel in mid-2021. The company had high hopes for the antibody at that point, pricing it at an average of $56,000 per year. Aduhelm…
Caribou Biosciences’ CEO discusses CRISPR progress, future goals, and gender equality in biotech
Founded in 2011, Caribou Biosciences is a pioneer in the development of CRISPR genome editing technologies, a field honored with the Nobel Prize in Chemistry in 2020. Co-founded by Jennifer Doudna, Ph.D., one of the Nobel laureates, and CEO Rachel Haurwitz, and two other CRISPR pioneers, the company has raised over $800 million in funding,…
Using AI to unlock new uses for existing cancer medicines
Repurposing is a drug development strategy that has been widely applied in cancer. This strategy, sometimes called label expansion, involves obtaining FDA approval to market a drug for the treatment of new indications, alone or in combination with other drugs. Not only can this approach extend the window of patent protection for a commercialized drug,…
MDMA’s potential shift from party drug to PTSD therapy could hinge on strict safety measures
MDMA, the stimulant mood-lifting drug commonly known as ecstasy, could soon transition from party staple to FDA-approved medication — but likely with tight control measures to address its abuse potential and safety risks. On Tuesday, the nonprofit Multidisciplinary Association for Psychedelic Studies (MAPS) filed an application seeking FDA approval of MDMA-assisted therapy for post-traumatic stress…
Cybin sees near future for psychedelic therapy after promising interim phase 2 data
With promising interim phase 2 data in hand, Cybin believes psychedelic therapy will become a reality in the “not too distant future,” according to CEO Doug Drysdale. As recently as the 1990s, it would be difficult to imagine that a psychedelic drug would potentially be a clinical option for a mood disorder like depression. But…
Lilly’s Zepbound to enter the weight management market with competitive pricing
Lilly’s tirzepatide notched an FDA approval for chronic weight management, potentially clearly the way for billions in additional sales. Analysts have projected that the drug could fetch $26 billion in annual sales by 2030, with roughly two-thirds of that sum related to obesity treatment. Bank of America analyst Geoff Meacham is even more optimistic, predicting…
Is the new $15,900 postpartum depression pill worth it?
Sage Therapeutics has pegged the wholesale acquisition price of the oral postpartum depression (PPD) drug Zurzuvae (zuranolone) at $15,900 for a 14-day course of the therapy. The drug, co-developed by Biogen, won the FDA green light in August. The companies plan on launching the drug in December. The DEA has classified zuranolone as Schedule IV,…
An overview of the RSV vaccine landscape: GSK aims to extend its approval of Arexvy?
GSK (NYSE:GSK) is aiming to expand the label for its respiratory syncytial virus (RSV) vaccine Arexvy, which was the first to win FDA approval. The firm is now eyeing an extension of the label to include adults aged 50 to 59, bolstered by encouraging preliminary data from a phase 3 study. The recent data from…
Eli Lilly’s obesity focus helps propel promising 2023
2023 is shaping up to be a blockbuster year for Eli Lilly. With the company’s stock soaring by almost 54% since the beginning of the year, analysts are largely upbeat about Lilly’s expanded focus on obesity and diabetes treatments. While the company has developed insulin for a century, the company is now broadening its horizons…
More than a century after its synthesis, MDMA could be headed for FDA approval for PTSD
First synthesized in 1912 by Merck, the empathogenic drug 3,4-Methylenedioxymethamphetamine (MDMA) is inching toward FDA approval following the positive results of a phase 3 study. The recently concluded phase 3 study, MAPP2, published in Nature Medicine, found that MDMA-assisted therapy significantly outperforms traditional talk therapy in reducing PTSD symptoms. Participants receiving MDMA-AT had an 86.5%…
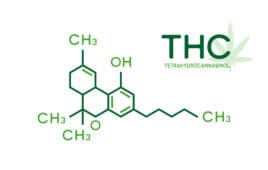
Anebulo wins positive FDA feedback to Advance phase 3 program for cannabis
The clinical stage biopharma Anebulo Pharmaceuticals recently shared that it received positive FDA feedback after a Type B meeting in July, where the regulatory agency indicated a path to approval for ANEB-001, a competitive CB1 antagonist with a high affinity for the human CB1 receptor. Overstimulation of this receptor by cannabinoids such as THC causes…
Legal dispute precede Pfizer’s latest FDA nod for RSV vaccine
Amid a backdrop of recent RSV vaccine approvals, GSK and Pfizer find themselves locked in a legal spat over alleged patent infringements. Both Big Pharma giants now possess the FDA’s blessing for their respective respiratory syncytial virus (RSV) vaccines. Pfizer’s recent win came with the second approval for its Abrysvo vaccine, which the company now…
Aiming to tame psychedelics’ wild side in pursuit of FDA approval
Doors of perception: Psychedelic renaissance or Pandora’s box? In one sense, psychedelics have always been divisive in mainstream Western culture. During their heyday in the 1960s, proponents lauded psychedelics’ virtues for psychological healing and exploration. Troubling reports also emerged — stories of bad trips, psychological breaks, and mostly apocryphal yet sensationalized reports of individuals leaping…
Key considerations for the FDA approval of zuranolone for postpartum depression treatment
Mixed-bag approval hit Sage hard but spared Biogen Sage Therapeutics recently notched an FDA win for the neuroactive steroid zuranolone (Zurzavae) for postpartum depression (PPD). For major depressive disorder (MDD), however, the agency handed the drug a complete response letter. In the wake of the news, Sage’s stock is down 46% to $19.52, while BIIB,…
FDA approves zuranolone for postpartum depression but issues complete response letter for major depressive disorder
FDA’s approval of Zurzuvae for PPD could mark a significant shift in the fight against postpartum depression (PPD). On August 4, the agency gave the green light for the oral medication, although it declined its use for major depressive disorder (MDD), citing insufficient evidence of effectiveness. The former approval represents a milestone as the first ever…
Leqembi could mark new era in Alzheimer’s treatment progress: An overview of the evolving drug development scene
Today, the FDA granted traditional approved to lecanemab (branded as Leqembi), a monoclonal antibody from Eisai and Biogen for adult patients with Alzheimer’s disease. The agency made the decision on the basis of a confirmatory trial that showed its clinical benefit. The drug, which reduces the formation of amyloid plaques in the brain, is the…
Insilico Medicine wins IND approval for AI-designed USP1 inhibitor for cancer trials in U.S. and China
Insilico Medicine has made a significant breakthrough with its AI-designed USP1 inhibitor, ISM3091. The US Food and Drug Administration (FDA) has accepted Insilico’s Investigational New Drug (IND) application for this promising drug, marking a significant milestone for AI-assisted drug discovery. “The FDA’s acceptance of our IND for ISM3091 signifies that the FDA recognizes its potential…
FDA approves expanded use of Farxiga (dapagliflozin) for heart failure patients regardless of ejection fraction status
FDA has approved an expanded indication for AstraZeneca’s Farxiga (dapagliflozin). This decision, marking a significant milestone in Farxiga’s development, opens up its use in patients with heart failure, regardless of their ejection fraction status. This brings the total number of Farxiga’s approved indications to five. Ejection fraction in heart failure Ejection fraction is a key measure…
Tirzepatide versus semaglutide: Which contender will prevail in the battle against obesity and type 2 diabetes?
Eli Lilly‘s (NYSE:LLY) tirzepatide achieved up to 15.7% weight loss in the SURMOUNT-2 study, sparking a potential tirzepatide versus semaglutide competition in the obesity and type 2 diabetes treatment markets. The phase 3 study enrolled 938 participants with diverse backgrounds. Tirzepatide promises to be a megablockbuster with a number of analysts pegging peak annual sales…
Amylyx Pharmaceuticals launches phase 2 trial for AMX0035 Wolfram syndrome therapy
Cambridge, Massachusetts–based Amylyx has dosed the first participant in its phase 2 HELIOS clinical trial of AMX0035 focusing on Wolfram syndrome. The condition is an ultra-rare genetic disorder involving the endocrine system. Symptoms of Wolfram syndrome can include diabetes insipidus, diabetes mellitus, optic atrophy and hearing loss. In September, Amylyx won FDA approval for AMX0035…
An overview of the RSV vaccine candidate landscape in early 2023
Respiratory syncytial virus (RSV) remains a prominent global health concern. Each year, the virus is to blame for 2.1 million outpatient visits for children under 5 years old, according to CDC. Other vulnerable populations, including older adults and immunocompromised individuals, are also at risk. Nevertheless, RSV has long been a research focus. Scientists first identified RSV in 1956.…