Over the past decade, the use of deeper sources of real-world data across all stages of the drug development life cycle has become increasingly important to guide disease understanding, trial designs, clinical guidelines, regulatory submissions and post-market studies. The advent of these deeper sources was prompted by the HITECH Act, which had the effect of…
How digital pathology can help drug developers address the forked road in oncology and beyond
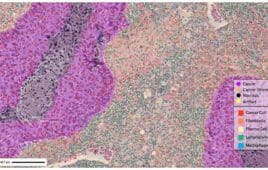
Digital pathology is disrupting the drug development process, replacing traditional microscopy rooted in Victorian Era approaches with high-resolution images and AI-powered analysis tools. In a recent editorial webinar, industry experts Nathan Buchbinder (chief strategy officer at Proscia), Dr. John Cochran (chief medical officer and chief pathologist at Q² Solutions, an IQVIA subsidiary), and Dr. Monika…
Inside the AI-powered Roche-PathAI companion diagnostics collaboration
PathAI and Roche Tissue Diagnostics (RTD) have inked an exclusive collaboration to develop AI-enabled companion diagnostics that builds on their initial partnership announced in October 2021. To date, PathAI and Roche have commercially launched four algorithms through the partnership. The new partnership will provide biopharma sponsors with integrated technology for developing companion diagnostics incorporating AI-based…
Unlearn CEO: Digital twins could slash clinical trial patient enrollment by 25% or more
The startup Unlearn embodies several trendy AI characteristics. Generative AI company? Check. San Francisco headquarters? Check. Aims to disrupt drug development (specifically, clinical trials) with AI? Check. Prominent AI leadership? Check. Mira Murati, the high-profile CTO of OpenAI, joined Unlearn’s board of directors in 2023. But the company’s pedigree is unique. The firm was founded…
In 2023, Roche and Novartis led the pack in drug pipeline scale
When reviewing R&D spending trends for 2023, Merck & Co. is a clear outlier given its decision to count its $10.3 billion Prometheus acquisition as an R&D charge. In all, the company committed more than half of its revenue to R&D. But Swiss giants Roche and Novartis remain frontrunners in terms of their pipeline of…
Could LSD change the game in anxiety treatment?
A once-controversial psychedelic substance could potentially be a promising treatment for generalized anxiety disorder (GAD). That’s the view of Dr. Rakesh Jain, a psychiatrist with extensive experience in clinical practice, research, and education, affiliated with Texas Tech University School of Medicine. Jain expressed optimism in LSD-based therapy while acknowledging the challenges inherent in such a…
Are Big Pharma giants getting the right ROI on their R&D investments? A visual exploration
While annual reports show broadly similar R&D strategies among Merck & Co., Pfizer, Johnson & Johnson and AbbVie, their 2020–2023 financial metrics reveal a concerning trend. Merck & Co. may be the new top dog of Big Pharma, but the firm’s 1.4% revenue growth in 2023 represents a significant slowdown. Pfizer’s 41.7% revenue decline from…
Moving the needle on diversity in clinical trials: Where do we go from here?
Enhancing patient diversity in clinical trials has become a key priority in drug development. The main concern is that critical data that includes underrepresented patient populations is being left out as many clinical trials do not reflect all populations that may eventually take a therapy. These underrepresented groups consist of women, including those who are…
From gatekeeper to strategist: The evolution of the CISO role in drug development
There’s an old joke about chief information security officers (CISOs) being gatekeepers of new technologies and initiatives – the infamous “Department of No.” Imagine a bouncer who, strangely, doesn’t let anyone in, saying the club is already too full, even when it’s clearly empty. But that image is outdated — especially in risk-focused industries like…
Off with the training wheels: AI-based patient characterization can improve clinical trial performance without large data sets
Only 12% of new drug candidates that enter phase 1 clinical development ultimately receive FDA approval. This dismal success rate leaves millions of patients with unmet medical needs and drives up the costs for the small number of drugs that make it to market. More frustratingly, it leaves untold numbers of potentially transformative therapies back-burnered…
Rice Biotech Launch Pad plans to make Houston a top-tier biotech hub
Houston boasts many world-class assets that have made it a formidable player on the global stage. From the world’s largest medical complex to mission control for the cosmos, few other cities can compete with its diverse strengths. Houston is also home to the prestigious Rice University, renowned for its leading science and engineering programs. Houston,…
Could an ‘extreme’ hibernating ground squirrel unlock new obesity treatments?
In late 2023, Eli Lilly, whose stock is now up close to 80% over the past year, inked a deal with the Emeryville, California–based Fauna Bio potentially worth $494 million that focuses on the discovery of novel drug targets for treating obesity. In 2020, Fauna entered into an obesity-focused collaboration with Novo Nordisk, Lilly’s primary…
Biotech bounces back at JPM 2024 on optimism, breakthroughs and calculated bets, but uncertainties persist
At the dawn of 2024, there’s a sense of renewed optimism in the biotech sector despite recent sector-specific challenges. This week, the JP Morgan Health Care Conference witnessed strong deal-making activity. For instance, Merck agreed to acquire cancer drug developer Harpoon Therapeutics for roughly $680 million, highlighting continued interest in oncology cancer therapies. Meanwhile, Novartis…
Core trends in 2023 FDA drug approvals: Oncology, neurology and hematology dominate
2023 was a big year for hematology, neurology and oncology, with the medical specialties seeing the most FDA approvals. In terms of sponsors, Pfizer had the most approvals with six total, followed by UCB and Chiesi, each with three apiece. When looking at commercial prospects, AstraZeneca’s respiratory syncytial virus antibody Beyfortus could be the biggest…
How a J&J exec found her calling in autoantibody drug development
Dr. Katie Abouzahr’s career, which began in the wards of the UK’s National Health Service (NHS) before extending into management consulting, paved the way for her leadership of autoantibody and maternal fetal medicine programs at Johnson & Johnson. “It’s not a typical pharma executive’s straight line path,” she acknowledged. “Careers can often be jungle gyms…
Two-thirds of pharma companies plan to up IT investments in 2024, survey finds
Two out of three pharma companies (67%) plan to ramp up investment in IT, including AI, over the next 12 months, according to a survey from the cloud vendor Rackspace Technology and Dell/VMware conducted in October 2023. About the same amount, 68%, reported challenges in recruiting and hiring talent skilled in cloud and AI technology.…
Unlocking generative AI requires reshaping culture, operations, and talent dynamics
In drug discovery and development, generative AI and natural-language processing (NLP) tools promise more than incremental productivity gains. For companies that can integrate such tools strategically into their workflows, the tools open the door to a fundamental rethinking of operational processes. For instance, generative AI tools can accelerate drafting of research articles, novel target identification,…
Cybin sees near future for psychedelic therapy after promising interim phase 2 data
With promising interim phase 2 data in hand, Cybin believes psychedelic therapy will become a reality in the “not too distant future,” according to CEO Doug Drysdale. As recently as the 1990s, it would be difficult to imagine that a psychedelic drug would potentially be a clinical option for a mood disorder like depression. But…
How Lantern Pharma and Code Ocean partnered on oncology drug development
A vision for data-driven drug development in oncology When Peter Carr, principal software architect of Lantern Pharma, stepped into his full-time role in September 2020, the company was on the cusp of a transformation. While AI had been a focus for a number of years, a fresh infusion of cash provided a possibility of expanding…
How the latest AI executive order might impact drug development in the U.S.
The White House has released an executive order that contains what it hails as “the most sweeping actions ever taken to protect Americans from the potential risks of AI systems.” Relevant to drug development, a fact sheet on the order describes its aim to help further “the responsible use of AI in healthcare and the…
A year in review: AI’s evolving role in drug discovery and development in 2023
In the realm of drug discovery, AI is making waves, and 2023 could potentially be a pivotal year for this technology. As the technology enters the popular consciousness, pharma employees are wondering “why they can’t have similar AI-driven tools for their professional tasks,” said Diane Wuest, head of digital R&D at Sanofi, in a recent…
Guselkumab shows durable benefits in Crohn’s disease in LTE of phase 2 study
The interleukin-23 blocker guselkumab (Tremfya) continues to show promise in treating Crohn’s disease (CD). First winning FDA approval for plaque psoriasis in 2017, guselkumab recently demonstrated robust efficacy and a consistent safety profile in the long-term extension of the GALAXI Phase 2 study for CD. Some 54.1% of patients receiving guselkumab achieved clinical remission by…
Alzheimer’s at an inflection point as drug and diagnostics breakthroughs emerge
Alzheimer’s disease research appears to be hitting its stride, thanks to recent therapeutic advances in drug development and the emergence of biomarkers to detect the condition. “All the pieces of the puzzle of precision medicine, which is already quite common in oncology, are now in place,” said Hartmuth Kolb, vice president, neuroscience biomarkers and R&D…
Moving beyond pilot purgatory: Scaling AI in drug discovery projects
The topic of AI in drug discovery and development may continue to garner significant attention, but harnessing AI effectively requires a nuanced strategy, and an ability to navigate between details and the bigger picture. Andrew Anderson, vice president of innovation and informatics strategy at ACD/Labs, likens it to boiling water. “On a small scale, it’s…
Johnson & Johnson pharma rebrand highlights innovation as a pillar to reinforce trust
Global pharma and medical device giant Johnson & Johnson (J&J) has ditched its iconic cursive logo that dates back to the late 19th century, and rebranded its Janssen pharma division as Johnson & Johnson Innovative Medicine. The move underscores the company’s push to prioritize higher-margin prescription drugs. This strategic move comes amidst a backdrop of…