Researchers from the NIH’s National Institute of Allergy and Infectious Diseases (NIAID) are conducting a clinical trial on allergic reactions to mRNA-based COVID-19 vaccines. The single-site trial will enroll up to 100 people between 16 and 69 years old who had an allergic reaction to a first dose of COVID-19 mRNA vaccines. NIAID seeks participants…
Medicago and GSK win approval from Health Canada for adjuvanted plant-based COVID-19 vaccine
GlaxoSmith Kline (NYSE:GSK) and Sanofi (NYSE:SNY) recently announced their intent to seek regulatory authorization for an adjuvanted recombinant protein-based COVID-19 vaccine. Now, GSK and its partner Medicago have received approval from Health Canada for another COVID-19 vaccine, which combines the former’s adjuvant technology with the latter’s plant-derived vaccine. Known as Covifenz, the vaccine makes use of plant-based virus-like…
Moderna skyrockets on Street-beating Q4 driven by COVID-19 vaccine sales
Moderna (NSDQ:MRNA) shares are on the rise on fourth-quarter results that came in ahead of the consensus forecast. MRNA shares were up 11.7% at $161.51 per share in midday trading today. MassDevice’s MedTech 100 Index — which includes stocks of the world’s largest medical device companies — was up 1.1%. The Cambridge, Massachusetts-based company posted…
Sanofi and GSK aim to commercialize COVID-19 vaccine
In the U.S., Moderna (NASDAQ:MRNA) and Pfizer (NYSE:PFE) continue to dominate the COVID-19 landscape while demand for Johnson & Johnson’s (NYSE:JNJ) vaccine remains limited. Now, Sanofi (NASDAQ:SNY) and GSK (NYSE:GSK) are preparing to get into the game by preparing their regulatory submissions for their COVID-19 vaccine. The companies are currently communicating with the FDA and…
FDA reportedly mulling authorizing fourth COVID-19 vaccine dose
Officials at the Food and Drug Administration are considering potentially authorizing an additional booster of the COVID-19 vaccines from Moderna (NASDAQ:MRNA) as well as Pfizer (NYSE:PFE) and BioNTech (NASDAQ:BNTX), according to the Wall Street Journal. At present, individuals 12 and older who are moderately or severely immunocompromised are eligible for four COVID-19 vaccine doses. Before the…
Pfizer-BioNTech omicron-based vaccine may be delayed
Pfizer (NYSE:PFE) and its partner BioNTech SE (NSDQ:BNTX) have launched a clinical trial to test an omicron-specific version of their COVID-19 vaccine. The two companies initially vowed to launch the updated vaccine by the end of March, but that goal may shift depending on the volume of clinical data regulators demand, according to BioNTech. In…
People who don’t get COVID-19 could provide clues for next-gen vaccines
A controversial SARS-CoV-2 challenge study involving 36 volunteers found that 47% of individuals did not develop COVID-19. The volunteers were unvaccinated and had no evidence of prior infection with SARS-CoV-2. The study, whose results were recently published in a pre-print, could potentially point to new vaccine targets or inspire future COVID-19 drug research. “This study has…
Pardes Biosciences shares interim Phase 1 data on oral COVID-19 antiviral
Pardes Biosciences (NSDQ:PRDS) said its PBI-0451 twice-daily COVID-19 pill candidate had favorable tolerability and good oral bioavailability, based on interim data from an ongoing Phase 1 trial. The company also said the drug candidate lacked clinically significant drug-drug interactions. “We’re very encouraged by the safety profile and bioavailability profile we’re seeing to date,” Brian Kearney,…
PharmaJet partner touts interim safety results for needle-free COVID-19 vaccine
PharmaJet announced today that its partner, Technovalia, reported positive interim safety results from a needle-free COVID-19 vaccine trial. Golden, Colorado-based PharmaJet’s needle-free injection systems are being studied with Covigen, a DNA-based vaccine developed by French-Thai pharmaceutical company BioNet-Asia in collaboration with Melbourne, Australia-based Technovalia. Enrollment for the trial began in June 2021. Get the full story at…
Omicron-specific mRNA vaccine elicited similar protection as original in early primate study
An NIH authored preprint concluded that an omicron-specific version of Moderna’s (NSDQ:MRNA) vaccine might not offer improved immunity or protection compared to the company’s current mRNA-1273 vaccine. In a small study involving macaques, NIH researchers tested neutralizing antibody levels and B cell expansion in primates receiving mRNA-1273 and mRNA-1273.529, the updated vaccine. The study involved a total…
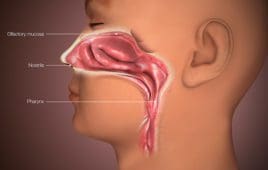
Could nasal vaccines be the next big weapon against COVID-19?
Because of the way they provide protection, nasal vaccines could be the best long-term way to prevent COVID-19 infection, according to experts cited in The New York Times. The Times reported that India-based Bharat Biotech, which has the Covaxin COVID-19 vaccine authorized in India and elsewhere, has an experimental COVID-19 nasal vaccine that may offer…
FDA could authorize Pfizer-BioNTech vaccine for young children in late February
Pfizer (NYSE:PFE) and BioNTech (NSDQ:BNTX) have begun a rolling submission for the BNT162b2 vaccine for children ages 6 months to 4 years. The FDA requested that Pfizer apply for emergency authorization for that age group, according to media reports. The agency is awaiting data from a third dose of the vaccine in the age group,…
Study bolsters case that vaccines protect against long-COVID
It may be too soon to know how often the omicron variant is associated with so-called long-COVID, but research from the Leavitt Partners’ COVID-19 Patient Recovery Alliance confirms that vaccination protects against the condition. Those who received at least one authorized COVID-19 vaccine dose before diagnosis were between seven and 10 times less likely to…
FDA fully approves Moderna’s COVID-19 vaccine
Moderna (NSDQ:MRNA) announced today that it received full FDA approval of the biologics license application for its COVID-19 vaccine. FDA’s approval for the company’s Spikevax mRNA COVID-19 vaccine covers the prevention of the virus in individuals aged 18 years or older. Moderna submitted for full FDA approval back in June 2021 and becomes the second…
Moderna commences Phase 2 study of omicron-specific booster
Mere days after rivals Pfizer (NYSE:PFE) and BioNTech (NSDQ:BNTX) announced the launch of a study testing an omicron-based booster vaccine candidate, Moderna (NSDQ:MRNA) has announced its own trial of an omicron-specific vaccine. The vaccine booster candidate, mRNA-1273.529, will be the subject of a Phase 2 study involving approximately 600 participants divided into two cohorts. The…
Pfizer and BioNTech test omicron-specific COVID-19 vaccine in adults
With COVID-19 cases continuing to hover near record levels, Pfizer (NYSE:PFE) and partner BioNTech SE (NSDQ:BNTX) have launched a clinical trial to test an omicron-based vaccine candidate in adults ages 18 to 55. The study will enroll 1,420 participants divided into three cohorts. The first cohort will have already received two doses of the initial…
Bharat Biotech’s Covaxin COVID-19 vaccine booster holds its own against omicron and delta variants
Bharat Biotech (Hyderabad, India) and its partner Ocugen (NSDQ:OCGN) have announced that their Covaxin (BBV152) COVID-19 vaccine candidate generated a strong neutralizing antibody response to the omicron (B.1.529) and delta (B.1.617.2) variants. The data comes from a study at Emory University involving sera from participants who received three doses of the vaccine with a 28-day…
BioNTech and InstaDeep use AI to predict high-risk SARS-CoV-2 variants
BioNTech (NSDQ:BNTX) and the enterprise AI company InstaDeep have announced that they have created an early warning system to improve the monitoring of potentially dangerous SARS-CoV-2 variants. The companies claim that the system spotted more than 90% of WHO-designated variants an average of two months before the organization officially classified them. For example, the system…
WHO concludes that updated COVID-19 vaccines may be needed
When confronting an onslaught of new SARS-CoV-2 variants, COVID-19 vaccine makers such as Pfizer (NYSE:PFE) and Moderna (NSDQ:MRNA) have tested tweaked versions of their vaccines but ultimately decided that they were unnecessary. Signs are growing that vaccines developed based on the original strain of the novel coronavirus will struggle to offer durable protection against the omicron…
Moderna and Pfizer look forward to fourth dose of COVID-19 vaccine
As COVID-19 case counts surge worldwide, executives at Moderna (NSDQ:MRNA) and Pfizer (NYSE:PFE) have voiced support for additional doses of their COVID-19 vaccines. Moderna CEO Stéphane Bancel predicted that a fourth dose would be necessary by the fall as the protection of vaccine-induced antibodies fades. In late December, Pfizer’s chief scientific officer Dr. Mikael Dolsten…
COVID-19 oral antiviral molnupiravir wins FDA nod
Merck (NYSE: MRK) and its partner Ridgeback Biotherapeutics have won emergency use authorization from FDA for the investigational oral antiviral molnupiravir (MK-4482, EIDD-2801). In late November, an FDA advisory panel narrowly voted in favor of authorizing molnupiravir. FDA recently also granted emergency use authorization to Pfizer’s (NYSE:PFE) oral SARS-CoV-2 antiviral Paxlovid (nirmatrelvir and ritonavir). FDA’s authorization of…
100-µg booster dose of Moderna’s vaccine increases antibody levels 83 times against Omicron
Moderna (NSDQ:MRNA) is touting preliminary data suggesting that its COVID-19 vaccine booster led to robust antibody increases against the Omicron variant, both at the 50 µg and 100 µg dose levels. The 50 µg booster dose, which is currently authorized under emergency use authorization, led to a 37-fold increase compared to pre-boost levels 29 days…
Sotrovimab wins EU marketing authorization as an early COVID-19 therapy
GlaxoSmithKline (LSE/NYSE:GSK) and its partner Vir Biotechnology (NSDQ:VIR) have received marketing authorization from the European Commission for the dual-action monoclonal antibody Xevudy (sotrovimab). The marketing authorization covers individuals 12 and older who weigh at least 40 kg who do not need supplemental oxygen. It also requires that such individuals have a heightened risk of developing…
Regeneron readies new COVID-19 monoclonal antibodies after the emergence of Omicron
Regeneron Pharmaceuticals (NSDQ REGN) plans to develop antibodies that hold up to the Omicron SARS-CoV-2 variant. Lab results from German researchers indicate that the company’s REGEN-COV/Ronapreve (casirivimab and imdevimab) antibody cocktail lost potency against the variant. REGN shares fell almost 4% to $631.74 today. “Our antibody cocktail works against Delta. Delta is still surging,” said Dr. Len…
Pfizer’s COVID-19 antiviral pill could be poised for European regulatory authorization
The Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has provided advice about using Pfizer’s COVID-19 antiviral Paxlovid. CHMP has concluded that Paxlovid (nirmatrelvir/ritonavir) could be used for adults with COVID-19 who don’t need supplemental oxygen and face an elevated risk of developing severe disease. CHMP also stressed the…