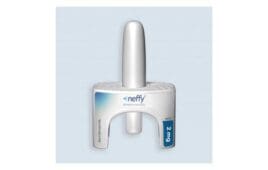
ARS Pharmaceuticals announced today that the FDA accepted its new drug application (NDA) for neffy, its intranasal epinephrine. The offering covers the emergency treatment of severe type I allergic reactions in children and adults weighing 30 kg (66 pounds) or more. The company set an anticipated target action date of mid-2023 for its PDUFA (Prescription…