
[ipopba/Adobe Stock]
In a related story, we share a timeline highlighting prominent examples of fraudulent activities in scientific research since the 1960s.
Given the alarming rise in suspicious medical research, we parsed through and visualized the retraction data from Retractiondatabase.org, focusing on retractions related to ethical violations from 2000 to 2022 related to the category “Health Sciences. The data were international in scope.
Falsification and fabrication in data and images in health sciences
The first graphic below illustrates the significant increase in cases within the “Falsification/Fabrication of Data,” “Falsification/Fabrication of Image” and “Paper Mill” categories in the database. Paper mills operate covertly, generating and marketing fraudulent scientific papers. These operations target researchers seeking to enhance their list of publications.
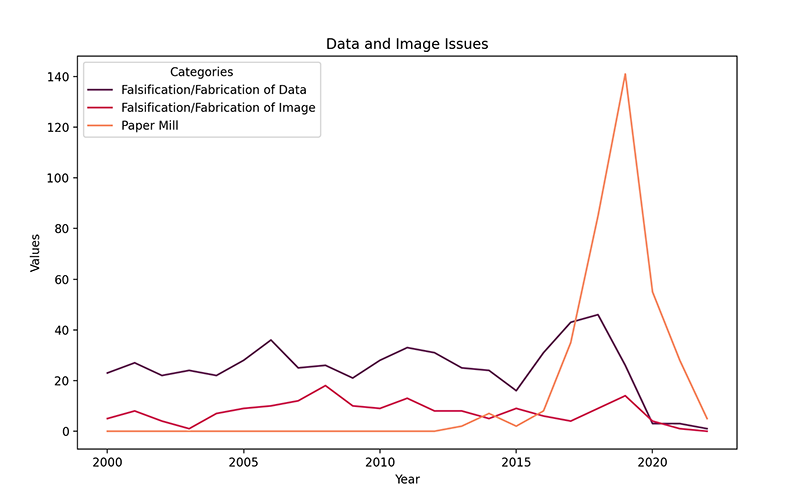
Figure 1: Trends in data falsification, image fabrication and paper mill issues from 2000 to 2022
The first graphic illustrates the significant increase in cases within the “Falsification/Fabrication of Data,” “Falsification/Fabrication of Image,” and “Paper Mill” categories in the database. Paper mills operate covertly, generating and marketing fraudulent scientific papers. These operations target researchers seeking to enhance their list of publications. The following line chart reveals a swift escalation in papers marked as originating from paper mills in 2017, with a prompt decrease following this peak. Rates of falsified data and images are relatively stable by contrast, although reports of fabricated data dipped in 2020 and held steady in the next two years.
Ethical violations, false authorship and criminal proceedings in health sciences
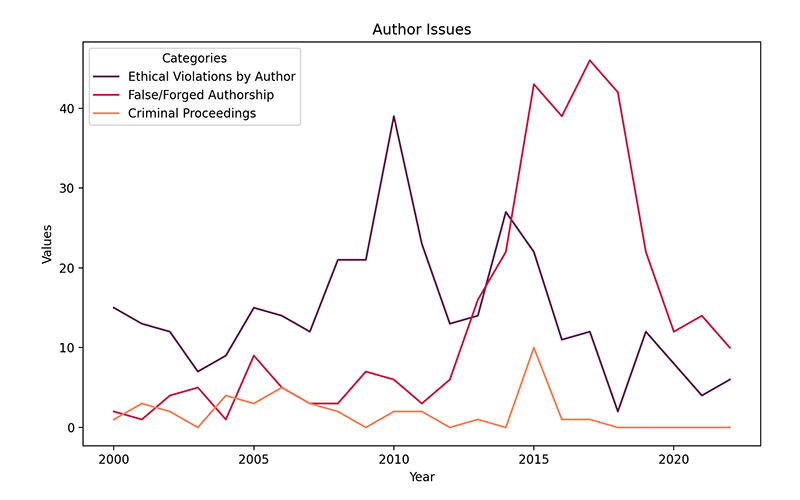
Figure 2: Instances of ethical violations by authors, false or forged authorship, and criminal proceedings from 2000 to 2022.
The second visual focuses on “Ethical Violations by Author,” “False/Forged Authorship,” and “Criminal Proceedings.” Data here paint a more complex picture, with various types of violations peaking and waning at different times. The category related to ethical violations by author peaked in 2010, with 39 instances. The category has generally fallen after that with only 6 cases in 2022. Roughly coinciding with the drop, however, was a rise in reports of false or forged authorship, which peaked in 2017 with 46 reports before falling to 10 reports in 2020.
Plagiarism and other trends in suspicious medical research
The final visual emphasizes the trends of plagiarism-related challenges categorized as “Plagiarism of Article,” “Plagiarism of Data,” and “Plagiarism of Text” in the database. The line chart reflects varying degree of these plagiarism reports over the past two decades. In particular, reports of plagiarized articles have risen significantly, hitting their peak around 2015 and then falling precipitously thereafter.
In contrast, reports of plagiarism of data and text cases fluctuate. Reports of plagiarized articles, similar to plagiarized text, also spiked in 2015. Many of the categories dipped in 2020, which could be a result of a range of factors including increased scrutiny and awareness as well as the impact of the COVID-19 pandemic.
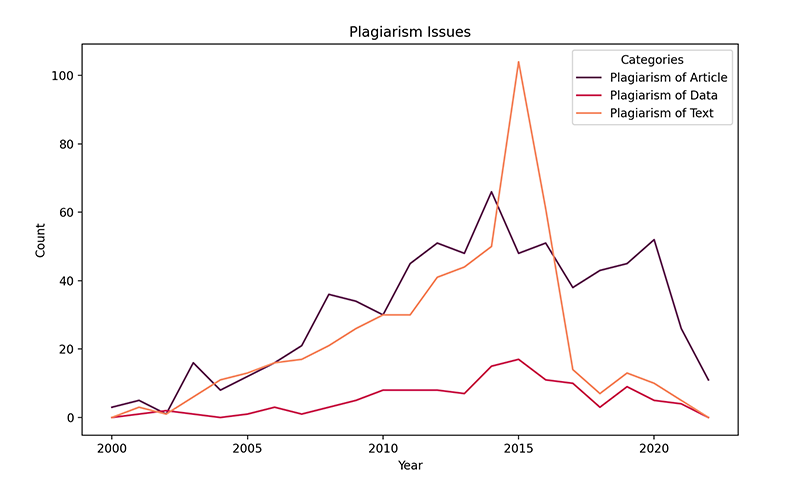
Figure 3: Changes in article, data, and text plagiarism cases from 2000 to 2022.
The need for more scrutiny of suspicious medical research
Despite improvements in some areas, data falsification and related problems remain persistent problems in scientific publishing. While retractions are necessary to help protect the integrity of scientific research, the scientific community must also explore additional strategies for identifying and dealing with suspicious medical research. The culture of “publish or perish” must evolve to emphasize the quality of scientific research over the quantity, discouraging the production of suspicious medical research.
Filed Under: Data science, Neurological Disease, Regulatory affairs




Tell Us What You Think!
You must be logged in to post a comment.