 Satsuma Pharmaceuticals (NSDQ:STSA) has announced that it has enrolled the first patient in its SUMMIT Phase 3 efficacy trial of STS101, a drug-device product that is a potential acute treatment of migraine.
Satsuma Pharmaceuticals (NSDQ:STSA) has announced that it has enrolled the first patient in its SUMMIT Phase 3 efficacy trial of STS101, a drug-device product that is a potential acute treatment of migraine.
STS101 is a nasal powder containing dihydroergotamine (DHE), an analgesic already available to treat migraines and cluster headaches. In fact, migraine patients have used DHE as an acute migraine treatment since 1946. But approved DHE products are “inconvenient or have an inconsistent response,” according to a paper published in Neurology. “Newer development-stage products may provide enhanced [pharmacokinetics], improved clinical efficacy, and more patient-friendly administration,” the article continued.
South San Francisco, Calif.–based Satsuma believes that STS101 could thus lead to higher efficacy over traditional dihydroergotamine-based nasal sprays.
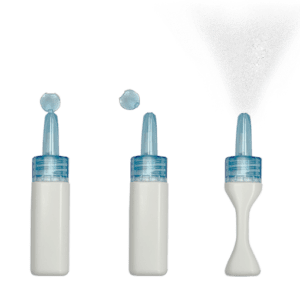
STS1 is the lead candidate of Satsuma Pharmaceuticals
“We believe the likelihood of success for SUMMIT is high given the utilization of the second-generation STS101 nasal delivery device and improved subject training in combination with the trial design and conduct adjustments we’ve made based on our analyses of results from the previous EMERGE Phase 3 trial,” said Satsuma President and CEO, John Kollins, in a statement.
The SUMMIT clinical trial will recruit approximately 1,400 participants.
The company’s EMERGE Phase 3 efficacy trial, however, failed to meet its primary endpoint, sending its share price down 84% in September 2020.
In a statement, the company explains that the SUMMIT trial will take into account learnings from the EMERGE study.
Volunteers in the SUMMIT trial will be randomized 1:1. The arm receiving STS101 will receive a 5.2-mg dose of the medicine.
There will be two primary endpoints of the trial — relief from pain two hours after treatment and freedom from other symptoms, including photophobia, phonophobia or nausea.
In 2019, the company said it would use proceeds form its $75 million IPO to pursue development of a nasal powder version of dihydroergotamine for migraine.
Filed Under: clinical trials, Drug Discovery, Neurological Disease





Tell Us What You Think!
You must be logged in to post a comment.