Findings from a large retrospective analysis presented at the European Lung Cancer Congress (ELCC) 2018, held 11 to 14 April in Geneva, Switzerland revealed that patients with advanced lung adenocarcinoma and complex epidermal growth factor receptor (EGFR) mutations demonstrated quite different response rates to standard first-generation tyrosine kinase inhibitor (TKI) treatment.
Patients with advanced lung adenocarcinomas can harbour complex mutations, such as two or more different EGFR mutations, within a single tumour sample, according to Bo Zhang of the Pulmonary Department, Shanghai Chest Hospital in Shanghai, China.
Whether the presence of complex mutations altered the efficacy of standard first-line treatment with TKIs was unknown. In addition, the frequency of complex mutations was also undocumented.
For this retrospective analysis, Dr. Zhang and colleagues recruited consecutive patients diagnosed with lung adenocarcinoma and an EGFR mutation from January 2011 to January 2017, and reviewed the efficacy of TKI treatment in patients with complex mutations.
Response to TKIs varies greatly according to EGFR mutation type
Of the 16,840 subjects that underwent screening, 5898 patients were positive for EGFR mutations. Complex mutations were diagnosed in 187 patients for a frequency of 3.2% in the EGFR mutant patients. Of these patients, 95 had advanced lung adenocarcinoma and were treated with TKIs.
While the 27 patients with adenocarcinoma with Del-19+21L858R mutation demonstrated an objective response rate (ORR) of 72.7% with TKI treatment, 28 patients with Del-19/21L858R+ atypical mutations had an ORR of 54.2%. Twenty patients with double atypical mutations had an ORR of 66.7% following TKI treatment.
Median progression-free survival (PFS) in the respective cohorts was 18.2 months (95% confidence interval [CI] 12.0, 24.4), 10.1 months (95% CI 6.5, 13.7), and 11.1 months (95% CI 6.8,15.4).
The poorest response was seen in 20 patients having tumours harbouring complex mutations with primary drug-resistant pattern who demonstrated an ORR of 15.0% with TKI treatment. Median PFS in these patients was just 1.4 (95% CI 0.2, 2.5) months.
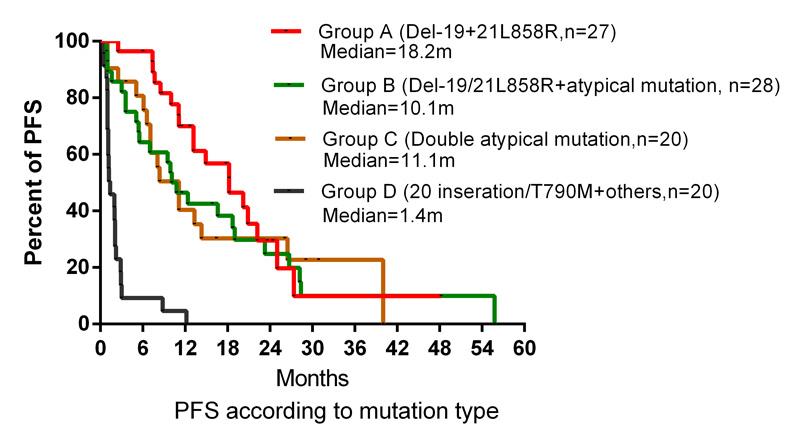
Dr Sandra Ortiz-Cuaran, who discussed the poster data, said that complex EGFR mutations account for 3.2% of the entire EGFR mutation spectrum. Patients with double classical mutations had the best PFS compared with other mutation types and may benefit more from TKIs treatment. Those who harboured complex mutations with classical pattern or double atypical mutations derived similar PFS compared with individuals who had single classical mutation.
Conclusions
The authors stated that first generation EGFR–TKI therapy is effective in patients with Del-19+21L858R, Del-19/21L858R+atypical mutations, and double atypical mutations. However, TKI treatment was less effective in patients with complex mutations and primary drug–resistant pattern.
Filed Under: Drug Discovery




