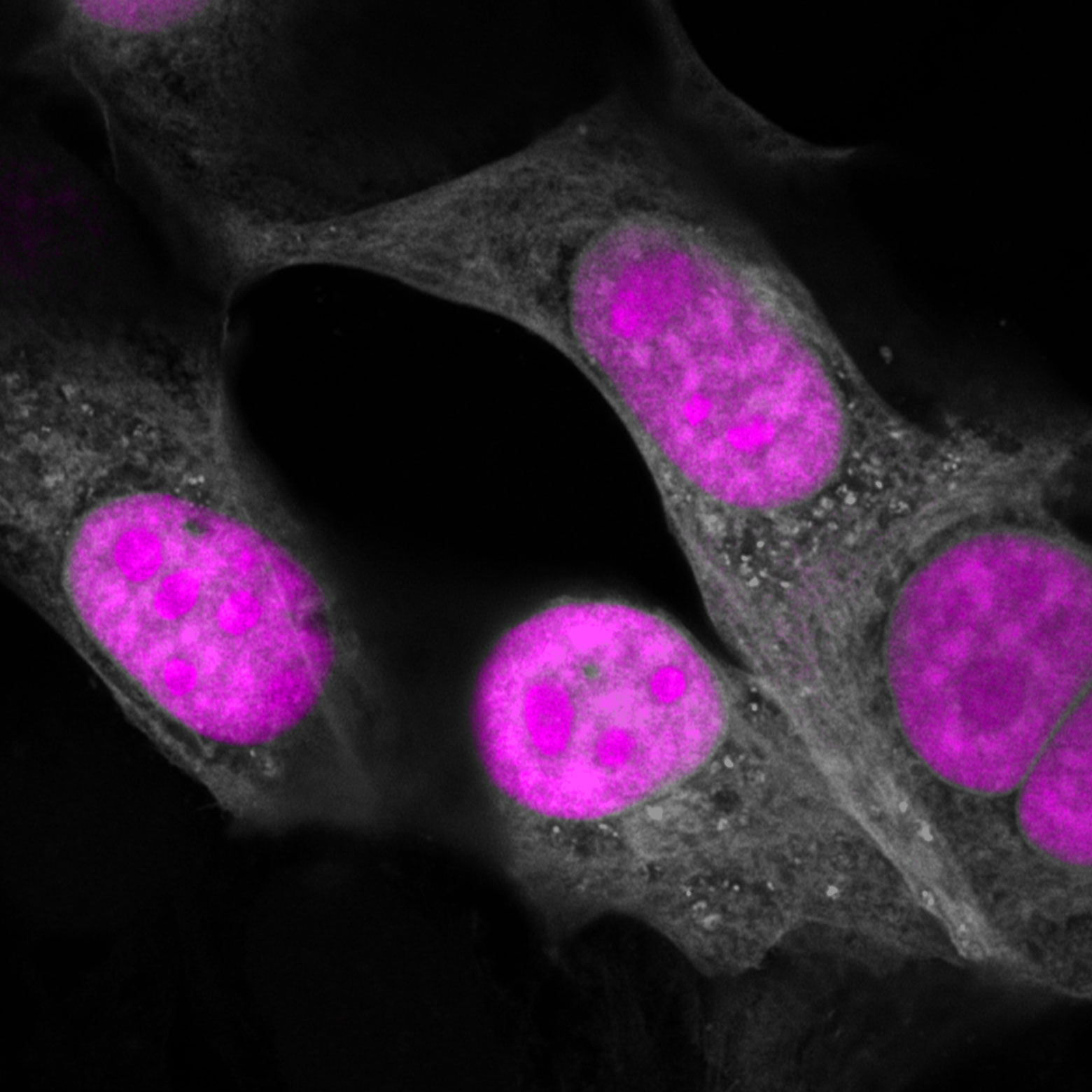
CasRx (magenta) targeting RNA in the nucleus of human cells (gray). Credit: Salk Institute
A team from the Salk Institute has created a new gene-editing tool that targets RNA and could correct a protein imbalance in the cells of dementia patients, restoring them to healthy levels.
CRISPR/Cas9 gene-editing technology has traditionally targeted DNA, acting as molecular scissors to cut and replace disease-causing genes with healthy ones.
The new tool, called CasRx, could increase the potential for genetic engineering of RNA and proteins, giving researchers a new method to develop new gene therapies, while also investigating fundamental biological functions.
“Bioengineers are like nature’s detectives, searching for clues in patterns of DNA to help solve the mysteries of genetic diseases,” Patrick Hsu, a Helmsley-Salk Fellow and senior author of the new paper, said in a statement. “CRISPR has revolutionized genome engineering, and we wanted to expand the toolbox from DNA to RNA.”
The researchers searched bacterial genomes for new CRISPR enzymes that could target RNA and ultimately be engineered to address issues with RNA and resulting proteins.
To do so, they developed a computational program that could search bacterial DNA databases for telltale signatures of CRISPR systems, including patterns of particular repeating DNA sequences, and found a family of CRISPR enzymes that targets RNA, which they called Cas13d.
Similar to the Cas9 family, Cas13d enzymes originate from different bacterial species that vary in their activity, enabling the researchers to identify the best version for use in human cells, Ruminococcus flavefaciens XPD3002, from the gut bacterium.
“Once we engineered CasRx to work well in human cells, we really wanted to put it through its paces,” Salk Research Associate Silvana Konermann, an HHMI Hannah Gray Fellow and the paper’s first author said in a statement said.
A given RNA message could be expressed at varying levels and its balance relative to other RNAs is crucial for healthy function. RNA could also be spliced in various ways to make different proteins. However, problems with splicing could result in diseases like spinal muscular atrophy, atypical cystic fibrosis and frontotemporal dementia (FTD).
“We began the project with the hypothesis that different CRISPR systems may have been specialized throughout an evolutionary arms race between bacteria and their viruses, potentially giving them the ability to target viral RNA,” Konermann said.
In FTD, the ratio of two versions of the tau protein is out of balance in neurons. The team genetically engineered CasRx to target RNA sequences for the version of FTD where tau protein is overabundant by packaging CasRx into a virus and delivering it to neurons grown from an FTD patient’s stem cells.
CasRx was 80 percent effective in rebalancing the levels of tau protein to healthy levels.
CasRx has advantages over other technologies that target RNA because it is small in size, highly effective and was created with no discernible off-target effects compared to RNA interference.
Filed Under: Genomics/Proteomics




