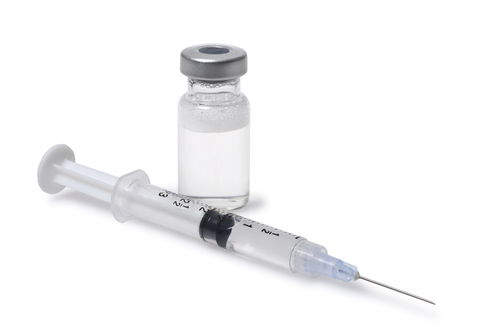
 The U.S. branch of Mylan N.V. announced Monday it would expand a voluntary nationwide recall of injectable drugs that are contaminated with foreign particulate matter.
The U.S. branch of Mylan N.V. announced Monday it would expand a voluntary nationwide recall of injectable drugs that are contaminated with foreign particulate matter.
Several groups of Gemcitabine and one lot of Methotrexate are the drugs being recalled. The first one can treat multiple forms of cancer whereas the second is intended for severe psoriasis and adult rheumatoid arthritis, as well as certain forms of neoplastic disease.
The company noticed the presence of the contaminant “during testing of retention samples,” according to the release. Both drugs belong to the class of sterile injectables, a type of medication capable of causing severe health problems if administered when infected with an unknown pollutant. A chance of stroke or respiratory failure are some of the adverse events Mylan cites in the memo.
FiercePharmaManufacturing reports Mylan began the initial recall in April of a cancer drug called carboplatin after finding that it contained foreign particulate matter.
Mylan notes so far there have been no reports regarding these serious reactions.
Filed Under: Drug Discovery




