 Direct-to-patient clinical trial company Medable (Palo Alto, California) has recently hired MaryAnne Rizk as its chief strategy officer. Rizk was IQVIA’s former senior vice president, digital R&D strategy.
Direct-to-patient clinical trial company Medable (Palo Alto, California) has recently hired MaryAnne Rizk as its chief strategy officer. Rizk was IQVIA’s former senior vice president, digital R&D strategy.
Rizk was also recently named a PharmaVoice 100 leader.
Rizk is also a graduate advisor and an adjunct professor for the school of engineering at the Stevens Institute of Technology (Hoboken, New Jersey), where she founded the Pharmaceutical Alumni Association and earned a doctorate in technology management, systems engineering.
In the following Q&A, Rizk touches on what inspired her to join Medable, her core goals and what inspires her.
Drug Discovery & Development: What inspired you to join Medable?
 Rizk: I came home. Medable is on a mission to get effective therapies to patients faster by transforming clinical drug development with disruptive technologies, which aligns with my mission. Medable’s culture truly aligns with my “Ikigai,” a Japanese concept which roughly translates to “reason for being.” [Ed note: See graphic below.]
Rizk: I came home. Medable is on a mission to get effective therapies to patients faster by transforming clinical drug development with disruptive technologies, which aligns with my mission. Medable’s culture truly aligns with my “Ikigai,” a Japanese concept which roughly translates to “reason for being.” [Ed note: See graphic below.]
My career is coming full circle as I work to empower — along with Medable — the patient by creating intelligent partnerships with like-minded people and innovative technologies.
- CVS: As a pharmaceutical technician, understanding the needs of patients and engagement with pharmacy.
- Merck: Rotation of Quality Center of Excellence from clinical to commercial teams.
- Medidata: Determine how software can drive efficiency.
- Oracle: How enterprise solutions can advance through outsourced CRO partners.
- IQVIA: CRO clinical experts providing monitoring improvements.
- Medable: Driving impact of technology solutions across sponsors, CRO sites and patients.
Medable’s digital platform streamlines design, recruitment, retention and data quality for decentralized trials, replacing siloed systems with integrated digital tools, data and interfaces to accelerate trial execution. Medable connects patients, sites and clinical trial teams to improve patient access, experience, and outcomes.
DD&D: What are your core goals at Medable?
Rizk: My core goals include expanding the company’s global strategic partnerships and innovation ecosystem with CROs, medical device companies, software and data partners. These partner initiatives will help Medable advance its vision of human-centered research, enabling remote access to clinical trials across geographies to ensure a diverse representation of people from varied economic and racial backgrounds.
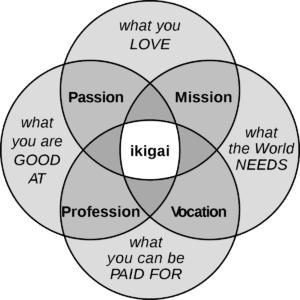
Image courtesy of Wikimedia Commons
DD&D: What inspires you?
Rizk: Medable’s “can-do” culture and patient-centered mission inspire me to do more and work harder every day. Driving transformation requires understanding how to build high-performing teams that are personally and professionally driven and building a network effect that expands on that culture. Finding your Ikigai is imperative to grow strategically. I’ve found mine.
Filed Under: clinical trials, Drug Discovery, Women in Pharma and Biotech





Tell Us What You Think!
You must be logged in to post a comment.