Austrian researchers have discovered that a small number of patients taking targeted drugs known as Janus kinase (JAK) inhibitors to treat myelofibrosis may develop aggressive lymphomas.
They also speculate that screening for a preexisting B-cell clone before starting therapy may help prevent this side effect and potentially save lives, according to a study published online today in Blood, the Journal of the American Society of Hematology (ASH).
One of three disorders that falls under the umbrella of myeloproliferative neoplasms is myelofibrosis, a rare bone marrow cancer in which too many blood cells are produced, leading to scarring and hardening inside the bone marrow. The exact cause of myelofibrosis is not known, but it has been linked to the JAK2 gene, which control the production of blood cells. Doctors treat the condition with medications designed to target or inhibit the action of this gene when it is faulty.
Though not a cure, JAK inhibitors are very effective at providing symptom relief, said study coauthor Heinz Gisslinger, MD, of the Medical University of Vienna in Austria. “However, we started noticing sporadic cases of lymphomas developing in patients being treated for myeloproliferative neoplasms and wanted to know if this phenomenon was connected to treatment.”
To test this, investigators assessed 626 patients receiving treatment for myeloproliferative neoplasms at the Medical University of Vienna and identified 69 that had myelofibrosis and were being treated with JAK inhibitors. Of those, four (5.8%) developed lymphomas. In comparison, they found that of the 557 patients who did not receive JAK inhibitors, only two (0.36%) developed lymphomas.
That amounts to a 16-fold increased risk for aggressive B-cell lymphoma in patients receiving JAK inhibitors, said study coauthor Ulrich Jäger, MD, of the Medical University of Vienna.
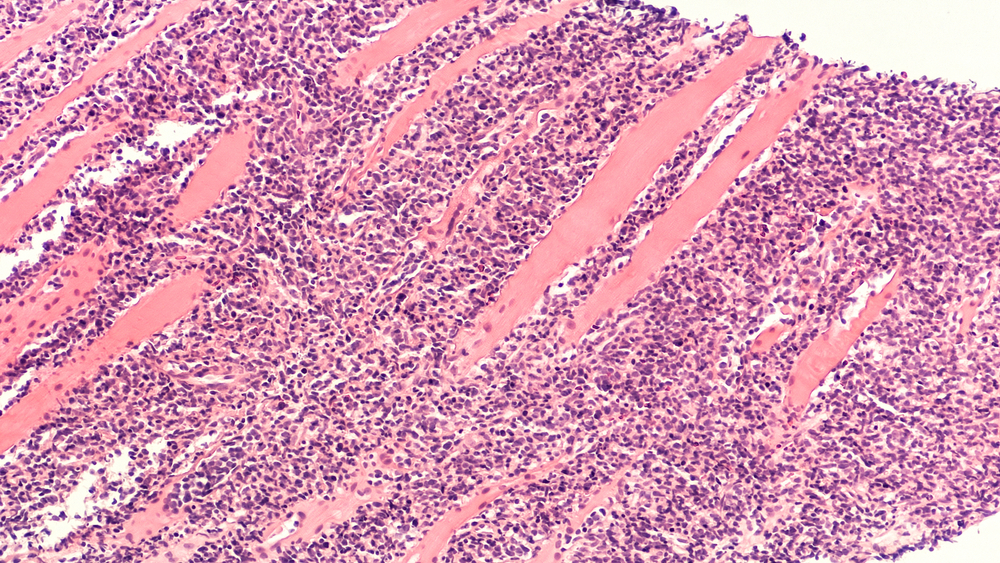
In samples taken from the patients with myelofibrosis, investigators found a preexisting B-cell clone in the bone marrow in three of the four patients who later developed lymphoma. Further investigation suggested that this clone was the same that later transformed into lymphoma.
The investigators also demonstrated an association between JAK inhibition and an increased frequency of aggressive B-cell lymphomas in mouse models.
“By replicating this link between this B-cell clone and aggressive lymphoma, we hope to speed the discovery of an alternative therapy for myelofibrosis,” said study coauthor Veronica Sexl, MD, of the University of Veterinary Medicine, Vienna. “These findings are going to be valuable in clinical care.”
“We determined that patients with this preexisting B-cell clone in their bone marrow are most at risk for developing aggressive lymphoma,” said Dr. Jäger. “We also know that up to 16 percent of people with myelofibrosis have immunoglobulin gene rearrangements like this B-cell clone. Therefore, our findings suggest that all patients with myelofibrosis should be tested for such gene rearrangements before prescribing JAK inhibitors to treat their disease.”
Filed Under: Drug Discovery




