 Ivy Brain Tumor Center and its partner Sonalasense have announced positive initial results in a first-in-human Phase 0/1 clinical trial involving recurrent glioblastoma patients.
Ivy Brain Tumor Center and its partner Sonalasense have announced positive initial results in a first-in-human Phase 0/1 clinical trial involving recurrent glioblastoma patients.
Sonalasense has developed a sonodynamic therapy (SDT) that pairs low-intensity ultrasound with chemotherapeutic agents known as sonosensitizers. The therapy is noninvasive.
Data from the trial indicate that SDT swiftly causes targeted oxidative stress and cell death in human glioblastoma tissue. In addition, the therapy was well tolerated in the study.
The Ivy Brain Tumor Center is using MRI-guided focused ultrasound in conjunction with Sonalasense’s 5-aminolevulinic acid (SONALA-001) to investigate the treatment. Investigators administered 5-aminolevulinic acid (ALA) intravenously, which then crosses the blood-brain barrier to enter the brain. Once in the brain, ALA targets glioma cells, which are abnormally metabolically active.
The study used intravenous aminolevulinic acid hydrochloride as an imaging agent to target the glioma with bursts of ultrasound.
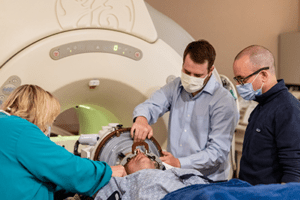
Shown here is the first patient-dosed with SDT
Participants in the study had histologically diagnosed grade III and IV gliomas.
Recurrent glioblastoma multiforme is an aggressive cancer form with an average survival time of 12 to 18 months, according to The Brain Tumour Charity.
“Sonodynamic therapy has tremendous potential to become a new nonsurgical therapeutic modality to treat brain tumors,” said Dr. Nader Sanai, who recently presented data from the trial at the European Society for Medical Oncology Congress. “We found that MR-guided focused ultrasound combined with SONALA-001 demonstrated rapid and selective cell death in the patients’ tumors, an encouraging discovery of this new noninvasive treatment option,” Sanai said in a statement.
Filed Under: clinical trials, Drug Discovery, Oncology





Tell Us What You Think!
You must be logged in to post a comment.