In the late 1800s, a New York surgeon named William Coley noticed that some patients with cancer seemed to fare better if they developed an infection after undergoing surgery. Suspecting that the immune system played a role in this mysterious response, he tried treating cancer patients with bacteria in an effort to turn on the immune system. Coley’s bold experiments largely failed, however, and faded into obscurity as other cancer treatments, such as radiation and chemotherapy, were put into practice.
Coley wasn’t wrong—just far ahead of his time. More than a century later, advances in how the immune system can be harnessed to fight cancer have offered new hope to patients with lung cancer. One of the latest immunotherapies to reach the market is a drug called nivolumab (Opdivo), which the FDA recently approved for the treatment of patients with advanced squamous non-small cell lung cancer (NSCLC).
“‘Groundbreaking’ and ‘revolutionary’” often overstate the case, but they truly apply to the impact of the new immunotherapy agents that target the PD-1 pathway for NSCLC,” says Naiyer A. Rizvi, MD, of the Herbert Irving Comprehensive Cancer Center and director of thoracic oncology and immunotherapeutics in the Department of Medicine at NewYork-Presbyterian Hospital/Columbia University Medical Center.
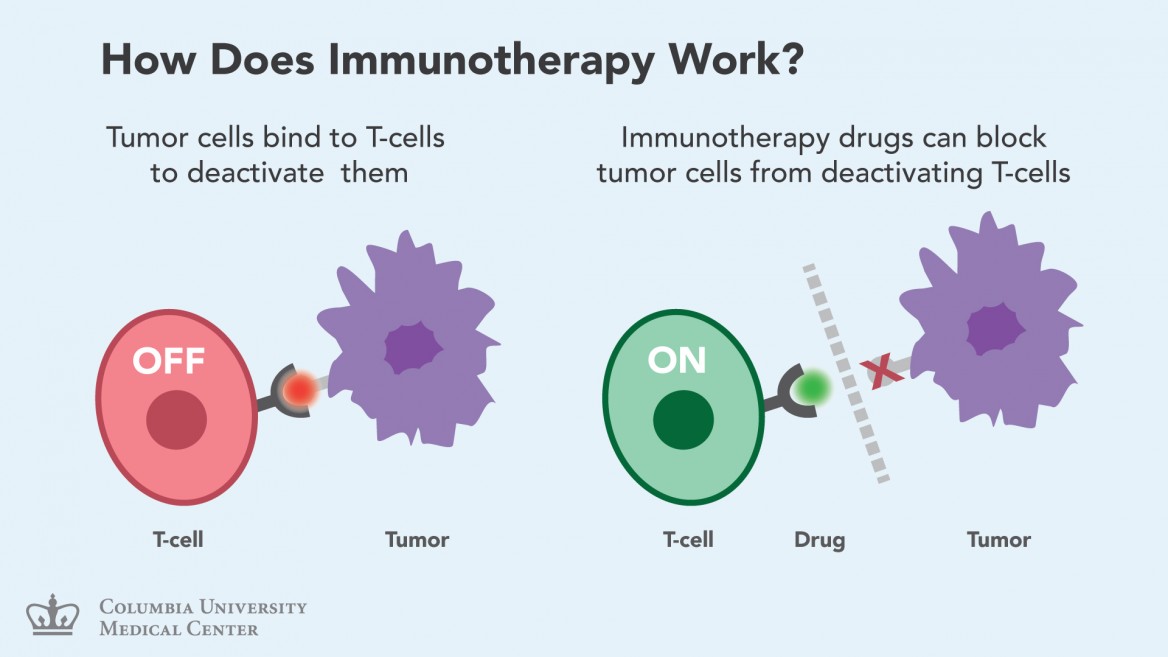
Nivolumab restores the immune system’s delicate balance by disabling the PD-1 protein on T cells. PD-1 works by suppressing T cell activity, so when this “checkpoint” protein is inhibited, T cells can go about their business.
Dr. Rizvi led a lung cancer trial recently published in Lancet Oncology that was key to approval of nivolumab for squamous lung cancer. “When I first started treating patients with nivolumab in 2008, it was hard to imagine how dramatically this could help patients who were resistant to all of our standard treatments,” says Dr. Rizvi. “We have some patients who are still alive many years after taking this drug, with no evidence of cancer. This has never been seen with standard lung cancer treatment.”
While some patients with NSCLC respond well to PD-1 inhibitors, others do not. Dr. Rizvi and his colleagues (at Memorial Sloan Kettering Cancer Center) thought that the cancers that had accumulated the most DNA damage were more likely to have worn out the immune system and would likely be helped the most by PD-1 inhibitors.
 They tested this by sequencing tumor DNA from both responders and non-responders to treatment with pembrolizumab (Keytruda), a PD-1 inhibitor. Among their findings, published in March in Science, was that patients with a great deal of DNA damage were far more responsive to treatment than those with less DNA damage. “We were able to use advances in sequencing technology to study the entire exome—the protein-coding genes of the genome—of tumors from patients with NSCLC who were treated with pembrolizumab. We found that the more genetically damaged the tumor was, the more likely the patient was to respond to PD-1 inhibitors.”
They tested this by sequencing tumor DNA from both responders and non-responders to treatment with pembrolizumab (Keytruda), a PD-1 inhibitor. Among their findings, published in March in Science, was that patients with a great deal of DNA damage were far more responsive to treatment than those with less DNA damage. “We were able to use advances in sequencing technology to study the entire exome—the protein-coding genes of the genome—of tumors from patients with NSCLC who were treated with pembrolizumab. We found that the more genetically damaged the tumor was, the more likely the patient was to respond to PD-1 inhibitors.”“This is an important first step toward being able to predict who will respond to PD-1 inhibitors and could be a new way to think about precision medicine based on the sequencing of tumor DNA,” says Dr. Rizvi. “This collaboration among clinical researchers, geneticists, and immunologists shows how a team of scientists can work together to help patients fight cancer.”
Papers discussed above:
“Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): a phase 2, single-arm trial,” published February 20, 2015, in the online edition of Lancet Oncology.
“Mutational Landscape Determines Sensitivity to Programmed Cell Death-1 Blockade in Non-Small Cell Lung Cancer,” published March 12, 2015, in the online edition of Science. Dr. Rizvi is a lead author of the paper.
Source: Columbia University
Filed Under: Drug Discovery




