
Cellares logo
The startup Cellares has a mission to enable industrial-scale cell therapy manufacturing with its Cell Shuttle, which it dubs a “factory in a box.”
The germ for the idea traces back to market research that South San Francisco, California-based Cellares co-founder and CEO Fabian Gerlinghaus focused on in a prior role. While attending industry conferences, Gerlinghaus stumbled upon traditional cell therapy manufacturing challenges and the opportunity to accelerate the process. “At these conferences, people were shouting from the rooftops, ‘We need cell therapy manufacturing technologies that are fully automated, fully closed and scalable to produce these life-saving therapeutics at a large scale for all the patients who need them,'” Gerlinghaus recounted.
Check out highlights from our interview with Cellares CEO Fabian Gerlinghaus:

Fabian Gerlinghaus
Cellares was born in April 2019 and has raised $100 million to date.
One of the chief objectives of the company’s Cell Shuttle, which provides end-to-end automation of cell therapy manufacturing processes, is to enhance repeatability and scalability.
How Cellares aims to change the cell therapy industry
The traditional process requires teams of highly trained professionals spending weeks in expensive cleanrooms executing dozens of manual processing steps on a plethora of benchtop instruments to serve a single patient. While the process enables pharma companies with flexibility, it also “locks them into a manufacturing paradigm that is very failure-prone,” Gerlinghaus said. “We’re seeing process failure rates of up to 18% in the cases of some of the cell therapy companies, and the cell therapies are very expensive to produce.”
Still, the industry is growing. Gerlinghaus said 2021 was “an incredible year for the cell therapy industry.” FDA approved several new cell therapy indications.
[This feature is a part of 2022’s Pharma 50 series.]
But it remains challenging to make cell therapies to meet the needs of tens of thousands of patients annually while optimizing quality and reducing the risk of operator error.
What sets Cellares apart
Gerlinghaus says Cellares is uniquely positioned to tackle the problem. “I’d say one of the most differentiating factors of the Cell Shuttle is that it automates not just one of the unit operations, but all unit operations,” he said. “We’re automating the entire cell therapy manufacturing process from start to finish. The Cell Shuttle does this not just for one manufacturing process at a time, but for ten manufacturing processes simultaneously.”
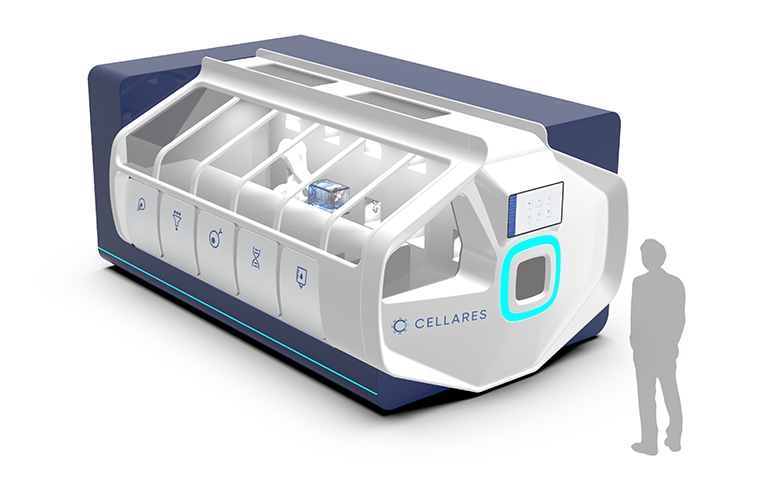
Cellares calls the Cell Shuttle a factory-in-a-box for cell therapy manufacturing.
Cellares made considerable progress in the technology development front in 2021 as it roughly tripled its number of employees from approximately 30 to 100, Gerlinghaus said, but said the company was not yet ready to share details about those technological advances.
Also last year, the company announced that it had added the bioethicist Dr. Ezekiel J. Emanuel to its board of directors.
Ultimately, Gerlinghaus says, “the combination of a manufacturing platform that provides end to end automation with an order of magnitude improvement in instrument throughput, is what makes this a scalable platform that pharma companies can use to meet commercial-scale patient demand [..] in a cost-efficient and robust manner.”
Filed Under: Cell & gene therapy, Video features





Tell Us What You Think!
You must be logged in to post a comment.