 The digital pathology software company PathPresenter (Montville, New Jersey) and the precision oncology platform firm 4D Path have entered into a partnership that could improve the diagnostic accuracy of breast tumor profiling.
The digital pathology software company PathPresenter (Montville, New Jersey) and the precision oncology platform firm 4D Path have entered into a partnership that could improve the diagnostic accuracy of breast tumor profiling.
PathPresenter has developed a digital workflow tool for clinicians, pathologists and pharma companies to aggregate medical data.
The platform can be used for medical education, patient care and management. In addition, pharma companies can use it to create models for drug development.
“For these workflow solutions to be effective, you need good AI models running in the background,” said Dr. Rajendra Singh, co-founder of PathPresenter. “And one of the best companies on the planet right now producing AI models is 4D Path.”
That observation ultimately led to the partnership between the two companies. “It was a natural fit to bring in their AI models into a workflow solution to provide a complete package to any clinician pathology department organization or a pharma company,” Singh concluded.
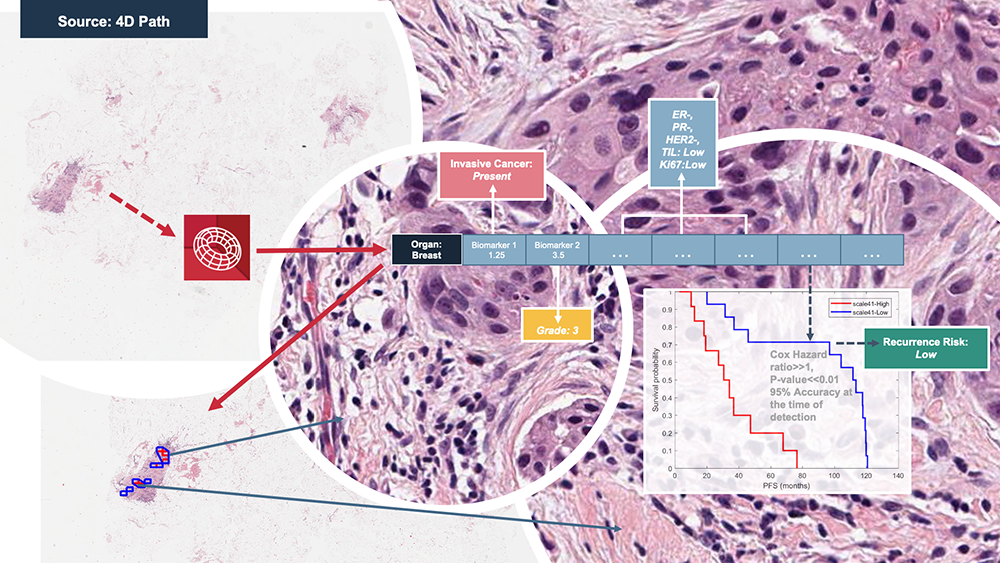
4D Q-plasia OncoReader Breast sample workflow and output. [Image courtesy of 4D Path]

Dr. Rajendra Singh
“We have built a solution to extract essential information from biopsy resection images that are routinely measured,” said Satabhisa Mukhopadhyay, co-founder and chief scientific officer of 4DPath. “Delivering that information in a useful and sensible way without disrupting workflow could be attractive.”
FDA granted breakthrough device designation to the 4D Q-plasia OncoReader Breast in 2020.
The alliance between the two companies resulted in integrating 4D Path’s 4D Q-plasia OncoReader Breast software within PathPresenter’s new clinical workflow platform, ClinPx.
The integration of 4D Path’s proprietary algorithms within the PathPresenter ClinPx platform could improve the throughput, reliability and quality of physician consultations.

Satabhisa Mukhopadhyay
Drug developers could benefit from the alliance to enable standardized central pathology review of certain biomarkers in oncology clinical trials and quantify tumor heterogeneity.
“Tumors can have a lot of heterogeneity. They can have different clonal populations,” Mukhopadhyay said. “So the molecular profile you are extracting can be very heterogeneous, which can, in turn, can affect responder risk to the drug with categories of responder, non-responder, and hyper-progressive. All of these are important for drug development.”
In any event, the rise of digital pathology could have a sweeping impact on the field of medicine at large. “For the past 100 years, pathologists have been looking at glass slides,” said Singh, a pathologist himself. “They would put a tissue slide under the microscope, look at the tissue and try to come to a diagnosis.”
The digitization of such slides in the past decade, however, is a gamechanger, Singh noted. “Instead of looking at a slide in a microscope, pathologists can see the same image in three dimensions on a computer screen,” he explained.
The sheer volume of digital slide data gives rise to increasingly powerful machine learning models. “Let’s say you have 500 breast cancer cases with 500 corresponding slides,” Singh said. “Experts at 4D Path can apply machine learning techniques to develop models that can predict the diagnosis.”
Such models can predict patient prognosis and make treatment recommendations for a given patient. In addition, 4D Path’s platform can also make predictions related to proteins such as estrogen receptors, progesterone receptors and the HER2/neu status.
“If a patient with breast cancer has a HER2/neu status of 3+, they could be given a specific drug,” Singh said. “But if it is 1+ or 2+, they might be given a different treatment.”
“If these algorithms are available for the pathologist to run when they’re looking at the slides on the computer, then they can actually help the clinician decide much faster what drug will be useful for the patient,” Singh concluded.
Rather than using standard AI, 4D Path uses statistical physics to uncover “hidden dynamics” in a frozen snapshot, Mukhopadhyay said. Deciphering information in those hidden dynamics can inform “what targeted drug you should provide or how a patient could respond,” she added.
The platform can also suppress noise obscuring the slide image.
The FDA or the EMA have not approved the 4D Q-plasia OncoReader Breast software for primary diagnosis. The company notes that the software is currently for research use only.
Filed Under: clinical trials, Drug Discovery and Development





Tell Us What You Think!
You must be logged in to post a comment.