 [Updated on February 23 to include information on new funding from California Institute for Regenerative Medicine. ]
[Updated on February 23 to include information on new funding from California Institute for Regenerative Medicine. ]
Osteoarthritis (OA), a degenerative joint disease affecting the knee and other joints, affects hundreds of millions of people worldwide.
Genascence is developing a gene therapy known as GNSC-001 for OA of the knee. A potent inhibitor of interleukin-1 (IL-1) signaling, GNSC-001 is a recombinant adeno-associated vector (AAV) with a coding sequence for interleukin-1 receptor antagonist (IL-1Ra).
The Palo Alto, California–based startup believes GNSC-001 could offer durable inhibition of IL-1 after a single injection.
“Gene therapy has been a big passion of mine,” Genascence CEO Tom Chalberg said in an interview. “And osteoarthritis is one of the few areas of medicine that hasn’t enjoyed any benefit of the biologics revolution.”

Tom Chalberg
Traditional knee OA treatment includes non-surgical strategies such as weight management, physical therapy and medications to reduce pain and inflammation. Physicians may treat more advanced knee OA with surgical options such as osteotomy or joint replacement.
“We’re poised to be the first gene therapy in osteoarthritis,” Chalberg said.
Genascence plans to begin a Phase 1b study of GNSC-001 involving approximately 50 patients. In May 2021, the company announced that GNSC-001 was safe and well tolerated in a Phase 1 single-arm, open-label, dose-escalation clinical trial of the gene therapy involving nine patients with knee OA.
One year later, Genascence announced it raised $10.5 million in Series A financing.
No larger unmet need
Part of the company’s vision is to extend the domain of gene therapy beyond rare disease to areas with major unmet need. “And there is no larger unmet need than osteoarthritis,” Chalberg said.
Genascence plans to optimize the potential cost-benefit ratio of GNSC-001 by enabling local delivery.
“We call it ‘focal delivery’ rather than systemic delivery,” Chalberg said. “That is an important approach in major diseases because it has a much lower cost of manufacturing, which makes it possible to bring this to market at a reasonable price.”
According to one estimate, OA costs the healthcare system $140 billion annually.
Although Tylenol, NSAIDs or steroids can help with symptoms, a central challenge in treating OA is that little can be done to reduce its progression. Joint replacement surgery can help many patients, but even in good cases, rehabilitation can take multiple months.
In addition, postoperative swelling and pain can persist for some time after a knee replacement. Approximately 15% of patients who underwent knee replacement surgery have residual moderate to severe pain that can last for up to five years after the procedure, according to an article in the Journal of Orthopedics and Orthopedic Surgery.
Targeting the major bad actor in OA
The potent inflammatory cytokine IL-1 plays a central role in the pathophysiology and development of OA. It destroys cartilage and prevents new cartilage formation. The original name for IL-1, catabolin, referenced its catabolic effects. Increasing amounts of IL-1 can amplify OA symptoms.
“Patients with high levels of IL-1 have worse pain, dysfunction and faster disease progression than patients with lower levels,” Chalberg said. “The good news is that you can block IL-1 with relatively modest levels of an inhibitor.”
Inhibiting IL-1 is not a novel goal. Several large double-blind, randomized controlled clinical studies targeting IL-1 have failed. Genascence believes that the improved delivery, which is local and long-term following a single injection into the joint, will be the key differentiator.
South Korea–based Kolon TissueGene is exploring a cell and gene therapy for osteoarthritis known as Invossa (TissueGene-C). In early 2022, TrialSpark announced that it acquired global rights to sprifermin, a recombinant form of human fibroblast growth factor 18 under investigation for OA, from Merck KGaA, Darmstadt, Germany.
In 2016, Vericel won FDA approval for MACI, a product based on a patient’s cartilage cells. MACI is the first FDA-approved autologous cellularized scaffold to repair cartilage defects in the knee.

Keravala
The academic founders of Genascence considered a strategy to deliver IL-1 blockade locally to the joint without the need for frequent intra-articular injections.
“That was basically the inception of GNSC-001, which is a recombinant adeno-associated viral vector that carries IL-1RN, which is a transgene that expresses interleukin-1 receptor antagonist (IL-1Ra),” said Annahita Keravala, co-founder and chief scientific officer of Genascence.
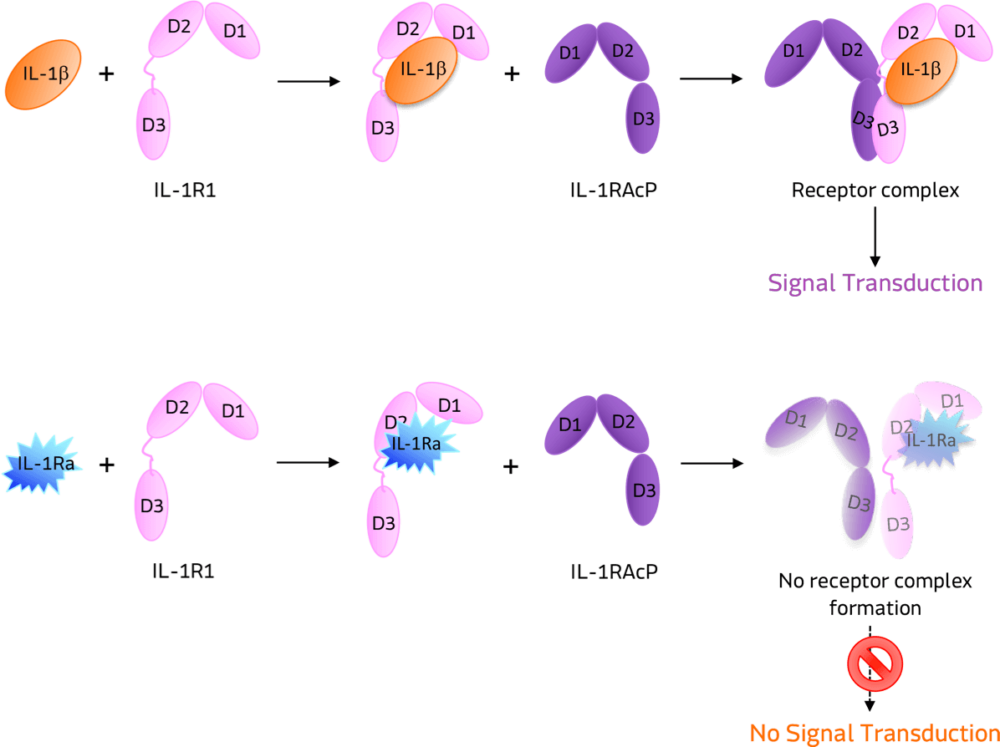
IL-1Ra is a potent inhibitor of IL-1. It binds to the same receptor as IL-1 and prevents the receptor from binding to its accessory protein and forming a reactive receptor.
“So when the receptor complex is not formed, IL-1 is unable to bind, thus inhibiting its downstream inhibitory effects,” Keravala said.
A little goes a long way
A small amount of IL-1RA has a potent effect in inhibiting IL-1.
“We believe that GNSC-001 is transformative for OA mainly because it is a single injection intra-articularly performed without any special device,” Keravala said.
The company envisions a physician administering the therapy with a standard 3 ml syringe.
[Image courtesy of Genascence]
In its preclinical research on horses, Genascence saw expression for 12 months in most animals — and 18 months in some cases.
Genascence says its AAV serotype not only transduces the synovial sites, the cells that line the joint capsule but can transduce chondrocytes, the cells that make up the cartilage.
In that acute study in horses, the company saw an improvement in movement and MRI scores at three months. Genascence then initiated a 12-month study where each arm had 11 horses. The study showed long-term expression of IL-1Ra.
“There was some variability, but every horse [in the treatment arm] had levels of IL-1Ra that were higher than baseline,” Keravala said.
The treated animals also showed improvements in movement, MRI scores and disease-modifying osteoarthritis drug (DMOAD) scores.
What’s next?
In February 2023, the company announced $11.6 million in funding from the California Institute for Regenerative Medicine (CIRM) to back the Phase 1b clinical trial and support manufacturing activities.
The Phase 1b study will test the safety and pharmacodynamics of GNSC-001 at two dose levels and test its effect on knee OA symptoms, biomarkers and disease progression.
“We applaud the Genascence team for tackling one of the most prevalent degenerative diseases,” CIRM President and CEO Dr. Maria Millan said when announcing the four-year award. “If successful, Genascence’s novel gene therapy approach could usher in a step-change in how we treat OA and other musculoskeletal diseases, which are among the leading causes of disability in California and around the world.”
Filed Under: Cell & gene therapy, Drug Discovery and Development





Tell Us What You Think!
You must be logged in to post a comment.